Abstracts
Introduction:
The fistulizing form of Crohn's disease (CD) represents a great challenge regarding treatment, especially perianal fistulas, for its high prevalence.
Objective:
To assess factors related to the response to surgical treatment associated with anti-TNF inpatients with CD and perianal fistulas.
Method:
Retrospective study of patients with CD and perianal fistulas who used IFX or ADA in association with surgical treatment.
Results:
30 patients with a mean age of 35 years were studied; 16 were treated with ADA (9 ADA + AZA) and 14 with IFX (10 IFX + AZA); ten of those treated with ADA responded, and of the six non-respondents, only one responded to IFX; eight responded to IFX, and among those non-respondents, no one showed response with ADA; among the respondents, there were 10 men and nine women; of those non-respondents, eight were men and 3 women; of those under 40 years, 16 responded compared with only three non-respondents; of those over 40 years, three responded versus eight non-respondents; as to the time elapsed between the onset of the disease and the beginning of anti-TNF, 14 (<2 years), one (2–5 years) and four (>5 years) responded, and five (<2 years), four (2–5 years) and two (>5 years) were non-respondents.
Conclusion:
There was no difference in response between the anti-TNF agents used; a better response was noted in those who used anti-TNF in combination with azathioprine, among women, in those under 40 years and in those treated within two years of the onset of the disease.
Crohn's disease; Infliximab; Adalimumab; Perianal fistula; Age
Introdução:
A forma fistulizante da Doença de Crohn (DC) apresenta um grande desafio quanto ao seu tratamento, especialmente as fístulas perianais pela sua alta prevalência.
Objetivo:
Analisar os fatores relacionados à resposta ao tratamento cirúrgico associado ao anti-TNF de pacientes com fístulas perianais por DC.
Método:
Estudo retrospectivo de pacientes com fístulas perianais por DC que fizeram uso de IFX ou ADA associado ao tratamento cirúrgico.
Resultados:
Foram estudados 30 pacientes com média de idade de 35 anos; 16 foram tratados com ADA (9 ADA + AZA) e 14 com IFX (10 IFX + AZA); 10 dos tratados com ADA tiveram resposta; e dos seis que não responderam, apenas um teve resposta com IFX; oito tiveram resposta com IFX; e dos que não responderam, nenhum apresentou resposta com ADA; dos que responderam, 10 eram homens e nove mulheres; dos que não responderam, oito eram homens e três mulheres; daqueles com menos de 40 anos, 16 responderam contra apenas três que não responderam; dos com mais de 40 anos, três responderam contra oito que não responderam; quanto ao tempo decorrido entre o início da doença e o início do anti-TNF, 14 (<2 anos), um (2–5 anos) e quatro (>5 anos) responderam, e cinco (<2 anos), quatro (2–5 anos) e dois (>5 anos) não responderam.
Conclusão:
Não houve diferença de resposta entre os agentes anti-TNF utilizados; houve melhor resposta nos que utilizaram anti-TNF em associação com azatioprina, entre as mulheres, nos pacientes com menos de 40 anos e naqueles tratados com até dois anos do início da doença.
Doença de Crohn; Infliximabe; Adalimumabe; Fístula perianal; Idade
Introduction
In itself, Crohn's disease (CD) constitutes a major challenge for the physician treating inflammatory bowel diseases. Those cases with fistulizing disease, particularly perianal fistulas, become even more challenging, as there is great difficulty in achieving its control in the long-term, even with the therapeutic advances made in recent years.11. Hardt MR, Kotze PG, Teixeira FV, Ludvig JC, Malluta EF, Kleinubing Jr H, et al. Epidemiological profile of 175 patients with Crohn's disease submitted to biological therapy. J Coloproctol (Rio J). 2012;32:395–401.
Today we know that the best therapeutic option for this presentation of CD is the association of surgical treatment with the use of anti-TNF agents, and in this sense, "surgical treatment" is not the classic fistulotomy or fistulectomy, but successive curettages and seton placements, allowing that the biologicals act in the healing process without formation of abscesses.22. Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: current management. JCC. 2010;4:28–62.
The conventional treatment of perianal fistulas in patients without CD produces excellent results; but in patients with CD one cannot employ the usual techniques in most cases, given the high probability of developing fecal incontinence. This situation changed positively with the emergence of biological agents, which significantly changed the treatment of this disease.
At the beginning of the use of biological agents, an increase in the incidence of perianal abscesses was also noted (because of the external orifice closure by the drug), but over time it was found that the combination of biologicals with frequent curettage of fistulas and seton placement constituted an effective strategy, having become the standard treatment for perianal fistulas in patients with CD.33. Assche GV, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. JCC. 2010;4:7–27.
What is being discussed today, in face of the inexistence of clear scientific evidence, is whether there are differences between the available biologicals, especially adalimumab and infliximab, which are the most used in Brazil. Thus, the aim of this study is to compare these two agents as to the differences in response to treatment with anti-TNF associated with surgical treatment in CD patients with perianal fistulas.
Objective
To assess factors related to the response to surgical treatment associated with anti-TNF (adalimumab and infliximab) of patients with perianal fistulas in Crohn's disease.
Method
A retrospective study of medical records of patients from the Inflammatory Bowel Diseases Outpatient Clinic, Hospital Universitario Maria Aparecida Pedrossian, Universidade Federal de Mato Grosso do Sul, from the Hospital Regional de Mato Grosso do Sul, and from the private practice of the author.
Patients with perianal fistulas and Crohn's disease with prescription and who made use of anti-TNF agents were included. All patients included were previously investigated for presence of TB and Hepatitis B.
The study period was from June 2000 to July 2013. Anti-TNF agents were used at recommended doses and intervals: infliximab (IFX) 5 mg/kg at weeks 0, 2 and 6 with maintenance every 8 weeks, and adalimumab (ADA) 160 mg at week 0,80 mg at week 2 and then 40 mg every 2 weeks.
All patients were treated by curettage and seton placement, with successive changes if necessary, until there was the possibility of removing the lesion. On average, the beginning of the biological therapy occurred one week after surgery.
Absence of pain and discharge for more than six months was considered as a satisfactory response to treatment.
The results were statistically analyzed by Student's t test and chi-squared test, and P <0.05 was considered statistically significant.
Results
30 patients (18 male and 12 female) were studied. The age ranged from 16 to 58 years, with a mean of 35 years.
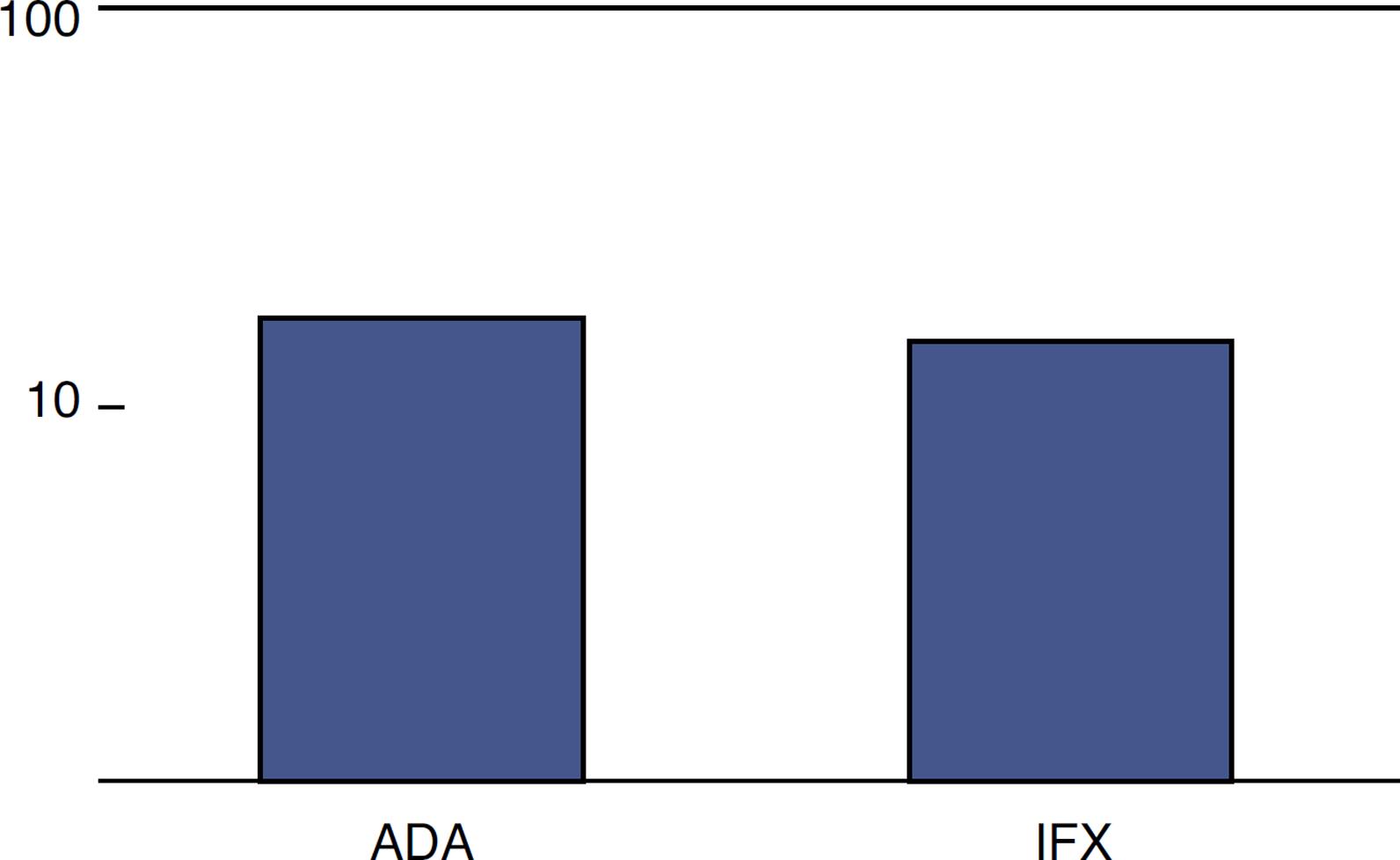
The choice of an anti-TNF agent was based on the availability and preference of patients, similarly to the data from the literature on the subject.44. Scarpato S, Antivalle M, Favalli EG, Nacci F, Frigelli S, Bartoli F, et al. Patient preferences in the choice of anti-TNF therapies in rheumatoid arthritis. Results from a questionnaire survey (RIVIERA study). Rheumatology. 2010;49:289–94.,55. Vavricka SR, Bentele N, Scharl M, Rogler G, Zeitz J, Frei P, et al. Systematic assessment of factors influencing preferences of Crohn's disease patients in selecting an anti-tumor necrosis factor agent (CHOOSE TNF TRIAL). IBD. 2012;18:1523–30. Of the 30 patients, 16 were treated with adalimumab and 14 with infliximab (Fig. 1).
The period of use of these anti-TNF agents ranged from 3 to 30 months, with a mean of 18 months.
Of those 16 patients using ADA, nine were also treated with azathioprine (AZA), while of those 14 who were treated with IFX, 10 also used AZA.
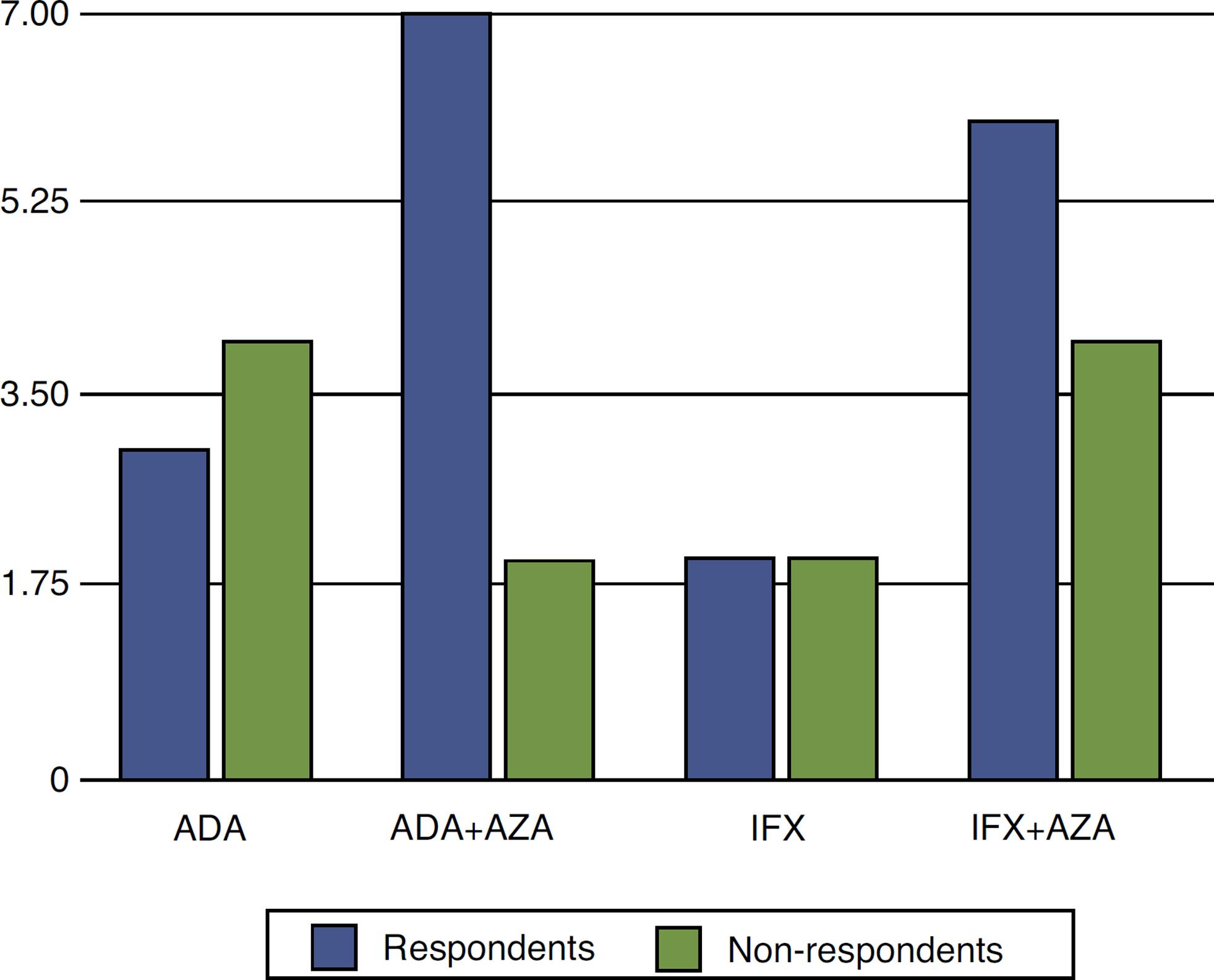
In the group treated with ADA, 10 had response and continued their treatment. Of the six non-respondents, three stopped its use altogether and three others started using IFX. Of these, only one had a response, remaining on this medication. In the group treated with IFX, eight responded. Of those six non-respondents, one stopped using anti-TNF and the other five started using ADA. Of these, no one had a response and the anti-TNF treatment was discontinued (Fig. 2).
Comparison between patients treated with infliximab and adalimumab, combined or not with azathioprine, in CD patients with perianal fistula.
There was no difference in our results when comparing anti-TNF agents (P = 0.22). However, it became evident that the combination of anti-TNF with AZA was superior versus monotherapy with this agent (P = 0.01).
When analyzing the results according to gender, it was observed that 10 men versus nine women (P = 0.13) responded, whereas eight men versus three women (P = 0.03) failed to respond (Fig. 3).
Comparison between genders as to response toanti-TNF therapy in patients with CD and perianal fistula.
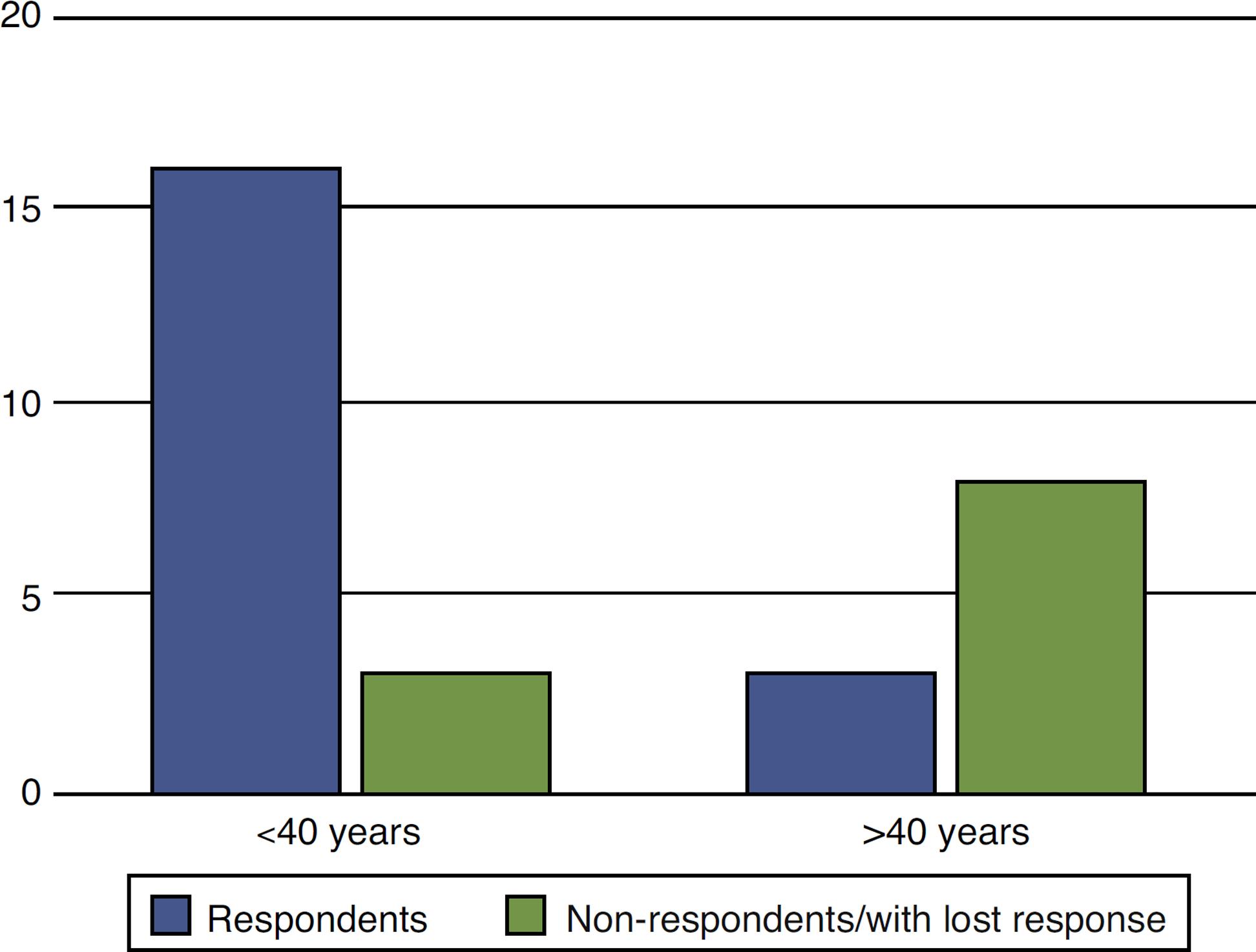
When the results of treatment of perianal fistulas according to age were analyzed, we observed that younger patients had better response versus older participants (P = 0.00002). Of those aged less than 40 years, 16 responded versus three non-respondents, while in those aged more than 40 years, only three responded versus eight non-respondents (Fig. 4).
Evaluation of response to anti-TNF in the treatment of anal fistulas in CD patients according to age.
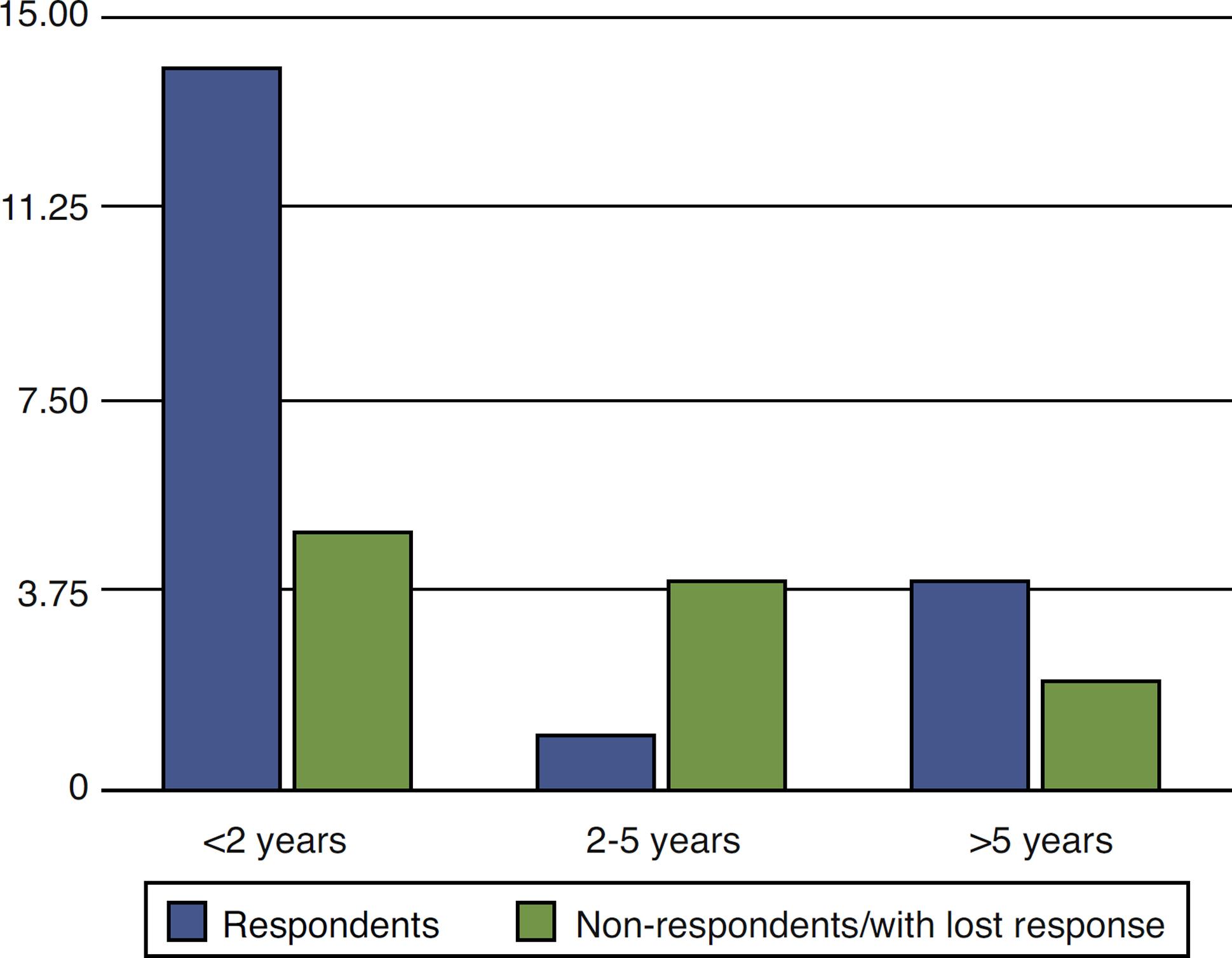
In the analysis of the response to treatment with anti-TNF in relation to the time elapsed between the diagnosis of perianal fistula in CD patients and the beginning of the therapy with the biological agent, it was found that 19 patients started anti-TNF therapy before two years of disease, five patients between two and five years, and six patients after more than five years of diagnosis.
Of those who started the treatment within two years of diagnosis, 14 responded versus five non-respondents/with lost response. Of those treated from two to five years of diagnosis, only one responded, while four were non-respondents/with lost response. In the group treated with anti-TNF more than five years after diagnosis, four showed no response versus two respondents (Fig. 5).
Evaluation of response to anti-TNF therapy in CD patients with perianal fistula in time elapsed between diagnosis and the use of a biological agent.
Discussion
A recent Brazilian publication on the epidemiology of CD showed that half of patients treated with the biological agent presented perianal disease, mainly perianal fistulas, high-lighting the importance of this form of the disease and the need for an effective treatment.11. Hardt MR, Kotze PG, Teixeira FV, Ludvig JC, Malluta EF, Kleinubing Jr H, et al. Epidemiological profile of 175 patients with Crohn's disease submitted to biological therapy. J Coloproctol (Rio J). 2012;32:395–401.
In a study of great relevance on this subject, Sands et al.66. Sands BE, Anderson FH, Bernstein CN, William WY, Feagan BG, Fedorak RN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876–85. observed that only 36% of their CD patients with fistulas maintained response after 54 weeks of IFX use. This demonstrates that, despite the great contribution given by the biologicals to the treatment of CD, particularly in the fistulizing variety, the majority of patients still do not achieve a satisfactory response with this therapy. Although the design of this article does not allow a direct comparison with the Sands et al. paper,66. Sands BE, Anderson FH, Bernstein CN, William WY, Feagan BG, Fedorak RN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876–85. we observed a complete response in 60% of cases.
Those individuals who show a good initial response to biologicals usually keep it over time.77. Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. This fact was also observed in the present study. The CHARM study also showed efficacy of ADA in 33% of cases of perianal fistulas at week 56, a finding very similar to that presented in the study of Sands et al.
Although the CHOICE study has shown encouraging results regarding the use of ADA in patients that failed with IFX, the same could not be observed in the present study. Lichtiger et al. observed that 39% of patients who had failed with IFX were successful in the healing of perianal fistulas and improved their quality of life. In our study, only one non-respondent patient with ADA was successful with IFX.88. Lichtiger S, Binion DG, Wolfà DC, Present DH, Bensimon AG, Wu E, et al. The CHOICE trial: adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn's disease who failed prior infliximab therapy. Aliment Pharmacol Ther. 2010;32:1228–39.
There are many publications on this subject, several of them of great consequence, but there is a shortage of articles evaluating other factors involved in the response to biological agents in CD patients with perianal fistulas. It seemed to us quite interesting to note that women had less treatment failures versus men with the use of biologicals. In the medical literature, there is little information on this subject.
In the present study we observed a better response in the younger group of patients, but there is no similar evidence in the literature. Weiss et al.99. Weiss B, Lebowitz O, Fidder HH, Maza I, Levine A, Shaoul R, et al. Response to medical treatment in patients with Crohn's disease: the role of NOD2/CRAD15, disease phenotype, and age of diagnosis. Dig Dis Sci. 2010;55:1674–80. found no difference in response to treatment with anti-TNF as to the age of patients in a recently published study, in which these authors analyzed this variable in relation to age group.
Currently, the time elapsed between the onset of symptoms of CD and the treatment with anti-TNF has been valued, since the shorter this time, the better the response to the therapy.1010. Panaccione R. Optimal use of biologics in the management of Crohn's disease. Therap Adv Gastroenterol. 2010;3:179–89. The same phenomenon was observed in the present study, given that the group treated at less than two years of diagnosis obtained better results than those treated within the period between two and five years. And this second group obtained better results than the group treated after five years of diagnosis.
Conclusion
There was no difference in response between anti-TNF agents used; there was a better response for those who used anti-TNF in combination with azathioprine, among women, in the group under 40 years and in those treated within two years of onset of the disease.
REFERENCES
-
1Hardt MR, Kotze PG, Teixeira FV, Ludvig JC, Malluta EF, Kleinubing Jr H, et al. Epidemiological profile of 175 patients with Crohn's disease submitted to biological therapy. J Coloproctol (Rio J). 2012;32:395–401.
-
2Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: current management. JCC. 2010;4:28–62.
-
3Assche GV, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, et al. The second European evidence-based consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. JCC. 2010;4:7–27.
-
4Scarpato S, Antivalle M, Favalli EG, Nacci F, Frigelli S, Bartoli F, et al. Patient preferences in the choice of anti-TNF therapies in rheumatoid arthritis. Results from a questionnaire survey (RIVIERA study). Rheumatology. 2010;49:289–94.
-
5Vavricka SR, Bentele N, Scharl M, Rogler G, Zeitz J, Frei P, et al. Systematic assessment of factors influencing preferences of Crohn's disease patients in selecting an anti-tumor necrosis factor agent (CHOOSE TNF TRIAL). IBD. 2012;18:1523–30.
-
6Sands BE, Anderson FH, Bernstein CN, William WY, Feagan BG, Fedorak RN, et al. Infliximab maintenance therapy for fistulizing Crohn's disease. N Engl J Med. 2004;350:876–85.
-
7Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
-
8Lichtiger S, Binion DG, Wolfà DC, Present DH, Bensimon AG, Wu E, et al. The CHOICE trial: adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn's disease who failed prior infliximab therapy. Aliment Pharmacol Ther. 2010;32:1228–39.
-
9Weiss B, Lebowitz O, Fidder HH, Maza I, Levine A, Shaoul R, et al. Response to medical treatment in patients with Crohn's disease: the role of NOD2/CRAD15, disease phenotype, and age of diagnosis. Dig Dis Sci. 2010;55:1674–80.
-
10Panaccione R. Optimal use of biologics in the management of Crohn's disease. Therap Adv Gastroenterol. 2010;3:179–89.
Publication Dates
-
Publication in this collection
Jul-Sep 2014
History
-
Received
25 Feb 2014 -
Accepted
15 May 2014