Abstract
Although the genus Duguetia is well known for producing alkaloids as chemical constituents, there are no reports of alkaloids identified in the species D. lanceolata. Thus, aiming to identify the chemical composition of this species, the dereplication of alkaloidic phase was performed by use of ultra-high performance liquid chromatography high resolution electrospray ionization tandem mass spectrometry (UHPLC-HR-ESI-MS/MS) and 1H nuclear magnetic resonance (NMR). The chromatographic fractionation of the alkaloid extract from Duguetia lanceolata (Annonaceae) leaves afforded four fractions (I-IV) that were shown to be composed of aporphine alkaloids. 1H NMR analysis and UHPLC-HR-ESI-MS/MS based dereplication allowed the identification of eight alkaloids: glaucine (1), norglaucine (2), isocorydine (3), N-methyllaurotetanine (4), oxoglaucine (5), liriodenine (6), lanuginosine (7), dehydroglaucine (8). Compounds 2,3, 4,6 and 7 were described for the first time in this species, while alkaloids 1,5 and 8 are newly discovered in the genus Duguetia. Additionally, the antiparasitic activity of the four fractions was evaluated in vitro against Leishmania infantum and Trypanosoma cruzi. Fraction I, composed exclusively by 1, displayed activity against Leishmania infantum and Trypanosoma cruzi intracellular amastigotes, with half maximal inhibitory concentration (IC50) values of 7.5 and 28.6 µg mL-1, respectively. Fraction IV (constituted by 2,3 and 4) showed activity against promastigotes of Leishmania infantum with IC50 value of 50.0 µg mL-1, while fraction II (constituted by 5 and 6) showed activity against trypomastigotes of Trypanosoma cruzi, with IC50 values of 83.0 µg mL-1. This work showed that fragmentation in UHPLC-HR-ESI-MS/MS combined with 1H NMR analysis of fractions is useful for identifying alkaloids in mixtures. Additionally, it was also demonstrated the potential of aporphine alkaloids from Duguetia lanceolata St. -Hil (Annonaceae) in the search for new drug candidates for neglected diseases.
Keywords:
Duguetia lanceolata
; Annonaceae; aporphine alkaloids; dereplication; UHPLC-HR-ESI-MS/MS; antiparasitic activity
Introduction
Annonaceae is composed of 135 genera and 2500 species. 11 Chatrou, L. W. ; Rainer, H. ; Maas, P. J. M. In Flowering Plants of the Neotropics; Smith, N. ; Mori, S. A. ; Henderson, A. ; Stevenson, D. W. ; Heald, S. V. , eds. ; The New York Botanical Garden: New York, 2004, p. 18. Among these genera, 34 can be found in South America and 29 occur in Brazil, including Duguetia. 22 Ribeiro, J. E. L. S. ; Hopkins, M. J. G. ; Vicentini, A. ; Sothers, C. A. ; Costa, M. A. S. ; Brito, J. M. ; Souza, M. A. D. ; Martins, L. H. P. ; Lohmann, L. G. ; Assunção, P. A. C. L. ; Pereira, E. C. ; Silva, C. F. ; Mesquita, M. R. ; Procópio, L. C. ; Flora da Reserva Ducke: Guia de Identificação das Plantas Vasculares de uma Floresta de Terra Firme na Amazônia Central; INPA, DFID: Manaus, 1999. The main chemical compounds isolated from Annonaceae are alkaloids, diterpenes, flavonoids, steroids and acetogenins. 33 Leboeuf, M. ; Cavé, A. ; Bhaumix, P. K. ; Mukherjee, B. ; Mukherjee, R. ; Phytochemistry
1982, 21, 2783.
4 Pérez, E. G. ; Cassels, B. K. In The Alkaloids: Chemistry and Biology, vol. 68, 1st ed. ; Cordell, G. A. , ed. ; Academic Press: London, 2010, p.83.
5 Lúcio, A. S. S. C. ; Almeida, J. R. G. S. ; da-Cunha, E.V. L. ; Tavares, J. F. ; Barbosa Filho, J. M. ; In The Alkaloids: Chemistry and Biology, vol. 74, 1st ed. ; Knölker, H. -J. , ed. ; Academic Press: London, 2015, p. 233.-66 Cavé, A. ; Leboeuf, M. ; Waterman, P. G. In Alkaloids: Chemical and Biological Perspectives, vol. 5, 1st ed. ; Pelletier, S. W. , ed. ; John Wiley: New York, USA, 1987, p. 133.
Duguetia lanceolata St. -Hil is popularly known as “pindaiva” or “ateira-da-mata”. 77 Isernhagen, I. ; Listagem Florística de Espécies Arbóreas e Arbustivas de Mato Grosso: Um Ponto de Partida para Projetos de Restauração Ecológica, vol. 1, 1st ed. ; Embrapa Agrossilvipastoril: Sinop, 2015. This species occurs in Brazil in the states of São Paulo, Minas Gerais, Mato Grosso do Sul, Paraná, Rio Grande do Sul and Santa Catarina. 88 Maas, P. J. ; de Kamer, H. M. V. ; Junikka, L. ; de Mello-Silva, R. ; Rainer, H. ; Rodriguésia 2001, 52, 61. Until now, there are few chemical reports about D. lanceolata in literature, and some studies describe identification of trans-asarone, 2,4,5-trymethoxystirene, stigmasterol, sitosterol and campesterol from its leaves. 99 Alves, D. S. ; Morejón, R. C. ; Machado, A. R. T. ; Carvalho, G. A. ; Pina, O. ; Oliveira, D. F. ; Semina: Ciênc. Agrár. 2015, 36, 4119.,1010 Gonçalves, G. L. P. ; Domingues, V. C. ; Ribeiro, L. P. ; Fernandes, J. B. ; Fernandes, M. F. G. ; Forim, M. R. ; Vendramim, J. D. ; Ind. Crops Prod. 2017, 97, 360. Furthermore, the chemical composition of its essential oil from bark and its biological activities has also been reported. 1111 Sousa, O. V. ; Soares Jr. , D. T. ; Del-Vechio, G. ; Mattosinhos, R. G. ; Gattass, C. R. ; Kaplan, M. A. C. ; Rev. Bras. Farmacogn. 2004, 14, 11.,1212 Sousa, O. V. ; Del-Vechio-Vieira, G. ; Alves, M. S. ; Araújo, A. A. L. ; Pinto, M. A. O. ; Amaral, M. P. H. ; Rodarte, M. P. ; Kaplan, M. A. C. ; Molecules 2012, 17, 11056.
Ultra-high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry (UHPLC-QTOFMS) has been widely used as an important tool for dereplication of natural product extracts, including alkaloidal fractions. 1313 Fabre, N. ; Claparols, C. ; Richelme, S. ; Angelin, M. L. ; Fourasté, I. ; Moulis, C. ; J. Chromatogr. A
2000, 35, 904.
14 Stévigny, C. ; Jiwan, J. L. H. ; Rozenberg, R. ; Hoffmann, E. ; Quetin-Leclercq, J. ; Rapid Commun. Mass Spectrom.
2004,
18, 523.
15 Carnevale Neto, F. ; Andréo, M. A. ; Raftery, D. ; Lopes, J. L. C. ; Lopes, N. P. ; Castro-Gamboa, I. ; Maia, B. H. L. N. S. ; Costa, E. V. ; Vessecchi, R. ; Rapid Commun. Mass Spectrom. , in press DOI: 10.1002/rcm.8533.
https://doi.org/10.1002/rcm.8533...
-1616 Pohlit, A. M. ; Oliveira, C. M. X. ; Lopes, J. L. C. ; Andreo, M. A. ; Niehues, M. ; Lopes, N. P. ; In Revisões em Processos e Técnicas Avançadas de Isolamento e Determinação de Ativos de Plantas Medicinais, vol. 1, 1st ed. ; Souza, G. H. B. ; Mello, J. C. P. ; Lopes, N. P. UFOP: Ouro Preto, 2012, p. 171. In addition, fragmentations observed by UHPLC high resolution electrospray ionization tandem mass spectrometry (UHPLC-HR-ESI-MS/MS) may be different from those observed with other mass spectrometric methods and it is important to understand these fragmentation mechanisms for the identification of such compounds. 1717 Nikolić, D. ; Gödecke, T. ; Chen, S. -N. ; White, J. ; Lankin, D. C. ; Pauli, G. F. ; van Breemen, R. B. ; Fitoterapia
2012, 83, 441.,1818 Sun, M. ; Liun, J. ; Lin, C. ; Miao, L. ; Lin, L. ; Acta Pharm. Sin. B
2014, 4, 208. Besides that, nuclear magnetic resonance (NMR) spectroscopy can be used to directly analyze compounds in mixtures. 1919 Lima, B. R. ; da Silva, F. M. A. ; Soares, E. R. ; de Almeida, R. A. ; da Silva-Filho, F. A. ; Barison, A. ; Costa, E. V. ; Koolen, H. H. F. ; de Souza, A. D. L. ; Pinheiro, M. L. B. ; J. Braz. Chem. Soc.
2020, 31, 79.
The aim of the present work was the characterization of alkaloids obtained from the leaves of D. lanceolata through dereplication by UHPLC-HR-ESI-MS/MS and 1H NMR. Additionally, the antiparasitic activity of fractions was evaluated in vitro against Leishmania infantum and Trypanosoma cruzi.
Experimental
Plant material
Leaves of Duguetia lanceolata St. -Hil were collected at the Atlantic Forest region in Alfenas, Minas Gerais, Brazil (21º22’53.8”S, 45º55’46.4”W). A voucher specimen has been identified and deposited at the herbarium of the Instituto de Biociências of the University of Sao Paulo (No. J.P.C.03.2017). The research project was registered in the Sistema de Patrimônio Genético e Conhecimento Tradicional Associado (SisGen) plataform, with registration No. A90708B.
General experimental procedures
All solvents were analytical reagents (AR) grade. Silica gel 60 F254 plates (Merck, USA) were used for comparative thin layer chromatography (TLC). TLC spots were visualized under UV light (254 nm) or using Dragendorff reagent for alkaloid detection. NMR spectra were recorded on a spectrometer Ultrashield 300 Bruker Avance III (USA, 300 MHz).
General HPLC parameters
HPLC analyses were performed in Dionex (Ultimate 3000 model, USA) equipped with a Kinetex column (EVO, 5 µm, C18 of 250 mm long × 4.60 mm internal diameter for analytical system), a spectrophotometric detector with a diode array (model UVD-17OU-DAD3000) and quaternary pump on analytical scale under the following conditions: gradient elution system, 15% MeOH (B) and 85% H2O acidified 0.1% formic acid (A) at 0.0 min; 15% B and 85% A at 2.0 min; 90% B and 10% A at 25.0 min; and 100% B and 0% A at 30 min. Flow was 1.0 mL min-1, sample concentration 2.0 mg mL-1, injection volume 20.0 µL and total run time 28.0 min.
UHPLC-HR-ESI-MS/MS experiments
UHPLC-HR-ESI-MS/MS data were acquired on a Shimadzu Nexera X2 system (Shimadzu, Japan) equipped with a SPD-M20A Proeminence diode array detector and coupled to a quadrupole time-of-flight mass spectrometer (MicroTOF-QII; Bruker Daltonics, USA) using an ESI ion source operating in positive ion mode. The applied nebulizer gas was 58 psi, flow and temperature of the drying gas (N2) were 9 mL min-1 and 220 ºC, respectively. Capillary voltage was set to 4500 V and end plate offset of 500 V. The mass analyzer was operated in SCAN mode in the range m/z 50-1200 and AutoMS mode with maximum of 5 precursors per cycle and collision energies varying from 15 to 70 eV. Accurate mass calibration was obtained using a solution of sodium formate (10 mM) as internal standard. Separation was performed applying a Kinetex columm (2.1 × 100.0 mm × 2.6 mm, Phenomenex) at 50 ºC using linear gradient of solvents starting from H2O:CH3CN (8.5:1.5) to H2O:CH3CN (1:9) in 12 min at a flow rate of 0.4 mL min-1. Both eluents were buffered with 20 mM of formic acid.
Extraction and semi-purification procedures
Dried leaves of D. lanceolata (150 g) were powdered and extracted with EtOH (5 × 500 mL at room temperature) affording (10 g) a crude extract after evaporation of the solvent under reduced pressure. Part of the EtOH extract (5 g) was dissolved in 3% HCl in water and then extracted with n-hexane. The aqueous phase (pH 1) was alkalized until it reached pH 10 with NH4OH and extracted with CH2Cl2. The CH2Cl2 phase was concentrated under reduced pressure to afford 1 g. The alkaloid phase (1 g) was chromatographed over silica gel eluted with CH2Cl2 containing increasing amounts of MeOH (up to 100%) to afford seven fractions (I-VII). Fraction I (4.0 mg), II (11.9 mg), III (10.8 mg) and IV (9.7 mg) showed positive results for Dragendorff reagent and were analyzed by HPLC. These fractions were subsequently analyzed by 1H NMR and UHPLC-HR-ESI-MS/MS allowing the identification of the alkaloids glaucine (1, tR = 15.1 min) from fraction I (Figure S2, Supplementary Information (SI) section); liriodenine (6, tR = 18.2 min) and oxoglaucine (5, tR =19.9 min) from fraction II (Figure S6, SI section); oxoglaucine (5, tR = 19.9 min), lanuginosine (7, tR = 22.4 min) and dehydroglaucin (8, tR = 25.4 min) from fraction III (Figure S12, SI section) and norglaucina (2, tR = 19.8 min), isocorydine (3, tR = 9.6 min) and N-methyllaurotetanine (4, tR = 18.1 min) from fraction IV (Figure S18, SI section).
Parasite maintenance
Promastigotes of Leishmania (L. ) infantum (MHOM/BR/1972/LD) were maintained in M-199 medium supplemented with 10% calf serum and 0.25% hemin at 24 ºC. Trypomastigotes of T. cruzi (Y strain) were maintained in LLC-MK2 (ATCC CCL 7) cells using Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with 2% calf serum at 37 ºC in a 5% CO2 incubator. 2020 Tada, H. ; Shiho, O. ; Kuroshima, K. : Koyama, M. ; Tsukamoto, K. ; J. Immunol. Methods 1986, 93, 157.
Antileishmanial activity
Promastigotes of Leishmania (L. ) infantum were seeded at 1 × 106 per well in 96 well microplates and incubated with different fractions for 48 h at 24 ºC. Then, the viability of promastigotes was measured by the mitochondrial activity using the colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. 2121 Pinto, E. G. ; Pimenta, D. C. ; Antoniazzi, M. M. ; Jared, C. ; Tempone, A. G. ; Exp. Parasitol. 2013, 135, 655.
Antitrypanosomal activity
Trypomastigotes of T. cruzi obtained from LLC-MK2 cells were seeded at 1 × 106 per well in 96-well microplates and incubated with the fractions for 48 h. The viability of trypomastigotes was determined using the colorimetric resazurin assay at 570 nm. 2121 Pinto, E. G. ; Pimenta, D. C. ; Antoniazzi, M. M. ; Jared, C. ; Tempone, A. G. ; Exp. Parasitol. 2013, 135, 655. Benznidazole was used as the standard drug.
Intracellular amastigotes of T. cruzi
Macrophages were collected from the peritoneal cavity of BALB/c mice by washing with RPMI-1640 medium supplemented with 10% FBS and maintained at 37 ºC in an atmosphere of 5% CO2 in a humidified incubator. Macrophages (1 × 105 cells per well) were dispensed in 16-well chamber slides (NUNC, Thermo, USA) and maintained for 24 h in the same medium at 37 ºC in a 5% CO2 humidified incubator for attachment. Non-adherent cells were removed by two-step washings with medium. After 24 h, these cells were infected with 1 × 106 culture trypomastigotes for 4 h (parasite-to-macrophage ratio 10:1). Subsequently, infected cells were incubated with fraction I at 100 to 0.78 µg mL-1 and with fractions II-IV at 200 to 0.39 µg mL-1 for 48 h. Finally, the slides were fixed with methanol, stained with Giemsa, and observed using light microscopy. The parasite load was defined by counting 200 macrophages per well using the formula: number of infected macrophages × number of intracellular amastigotes per total number of macrophages. 2222 Ferreira, D. D. ; Mesquita, J. T. ; Silva, T. A. C. ; Romanelli, M. M. ; Batista, D. G. J. ; da Silva, C. F. ; da Gama, A. N. F. ; Neves, B. J. ; Melo-Filho, C. C. ; Soeiro, M. N. C. ; Andrade, C. H. ; Tempone, A. G. ; J. Venomous Anim. Toxins Incl. Trop. Dis. 2018, 24, 30.
This study was approved by the Research Ethics Committees on Animals of Instituto Adolfo Lutz under No. CEU-IAL 05/2018.
Intracellular amastigotes of L. (L. ) infantum
Murine peritoneal macrophages were collected as described above and were seeded at 1 × 105 per well and incubated for 24 h in a 16-well slide chamber (NUNC, Thermo, USA). Amastigotes were obtained from the spleens of previously infected hamsters by differential centrifugation and seeded at a 1:10 ratio (macrophage: amastigotes) and kept at 37 ºC in a 5% CO2-humidified incubator for 24 h. Fraction I (at 100 to 0.78 µg mL-1) and fractions II-IV (at 200 to 0.39 µg mL-1) were incubated for 96 h with infected macrophages. Subsequently, the cells were fixed with MeOH, stained with Giemsa (Merck KGaA, Darmstadt, Germany), and observed using a light microscope. The parasite load was defined by counting 200 macrophages per well using the formula: number of infected macrophages × number of intracellular amastigotes per total number of macrophages. 2323 Wulsten, I. F. ; Costa-Silva, T. A. ; Mesquita, J. T. ; Lima, M. L. ; Galuppo, M. K. ; Taniwaki, N. N. ; Borborema, S. ; Costa, F. B. ; Schmidt, T. J. ; Tempone, A. G. ; Molecules 2017, 22, 685.
Results and Discussion
The EtOH extract from leaves of D. lanceolata was submitted to an acid-base extraction, according to literature, in order to afford alkaloidic phase. 2424 Carollo, C. A. ; Siqueira, J. M. ; Garcez, W. S. ; Diniz, R. ; Fernandes, N. G. ; Nat. Prod. 2006, 69, 1222. After column chromatography separation procedure, the Dragendorff positive fractions (I-IV) were analyzed by 1H NMR and UHPLC-HRMS/MS and allowed the identification of eight aporphinoid alkaloids, including four aporphine sensu stricto: glaucine (1), norglaucine (2), isocorydine (3) and N-methyllaurotetanine (4), three oxoaporphines: oxoglaucine (5), liriodenine (6) and lanuginosine (7), and one aporphine derivative dehydroglaucine (8) (Figure 1).
Preliminar NMR analyses in mixture of alkaloids
1H NMR spectrum of fraction I showed four singlets of methoxyl protons at δH 3.63, 3.85, 3.86 and 3.88 (Figure S1, SI section). Multiplets between δH 2.55-3.04 with integration for six hydrogens (H-4 and H-5, H-7) can be assigned to hydrogens of rings B and C of aporphine alkaloids and a singlet at δH 2.55, with integration for three hydrogens was assigned to hydrogens of the methyl group linked to nitrogen atom. Furthermore, three singlets at 6.71, 6.91 and 7.99 can be attributed to aromatic hydrogens H-3, H-8 and H-11, respectively, (Figure S1, SI section). This data made the identification of glaucine (1) (C21H25NO4) [M + H]+ 356.1861 (0.0 ppm) possible after comparison of 1H NMR data with those reported in literature. 2525 Huang, W. J. ; Chen, C. H. ; Singh, O. V. ; Lee, S. L. ; Lee, S. S. ; Synth. Commun. 2002, 32, 3681.
Fraction II was composed of two substances as indicated by HPLC retention times of 18.1 and 19.9 min (Figure S6, SI section). The 1H NMR spectrum of this mixture showed duplicated characteristic signals of oxoaporphine alkaloids, including two singlets at δH 7.39/7.51, attributed to H-3, besides aromatic hydrogens with two doublets at δH 7.74/7.79 and two at δH 8.87/8.92 that could be attributed to H-4 and H-5, respectively, of two different alkaloids (Figure S5, SI section). Also, two singlets at 8.03 and 8.75 were observed that can be attributed to H-8 and H-11 for one of the alkaloids. 2626 Kan, S. T. ; Ahmad, M. S. ; Azziz, S. S. S. A. ; Awang, K. ; Mhd Bakri, Y. ; Nafiah, M. A. ; Abbas, P. ; Hashim, Y. Z. H. -Y. ; Res. Pharm. 2019, 23, 217. In addition, the 1H NMR spectrum showed four aromatic protons at δH 8.47 (1H, m), 7.63 (1H, m), 7.82 (1H, m) and 8.56 (1H, m) which were attributed to the protons of the unsubstituted D ring of the aporphine nucleus like liriodenine. 2727 Hsieh, T. J. ; Wu, Y. C. ; Chen, S. C. ; Huang, C. S. ; Chen, C. Y. ; J. Chin. Chem. Soc. 2004, 51, 869.,2828 Wijeratne, E. M. K. ; Hatanaka, Y. ; Kikuchi, T. ; Tezuka Y. ; Gunatilaka, A. A. L. ; Phytochemistry
1996, 42, 1703. There was also one singlet at δH 6.46 corresponding to the presence of one methylenedioxy group, OCH2O and four methoxy signals with integration for three hydrogens each at δH 4.03, 4.08, 4.09 and 4.11 (Figure S5, SI section). Comparing with literature these signals may indicate the presence of oxoaporphine alkaloids 5 and 6. 2626 Kan, S. T. ; Ahmad, M. S. ; Azziz, S. S. S. A. ; Awang, K. ; Mhd Bakri, Y. ; Nafiah, M. A. ; Abbas, P. ; Hashim, Y. Z. H. -Y. ; Res. Pharm. 2019, 23, 217.,2929 Harrigan, G. G. ; Gunatilaka, A. L. ; Kingston, D. G. ; Chan, G. W. ; Johnson, R. K. ; Nat. Prod. 1994, 57, 68.
30 Rabêlo, S. V. ; Costa, E. V. ; Barison, A. ; Dutra, L. M. ; Nunes, X. P. ; Tomaz, J. C. ; Rev. Bras. Farmacogn.
2015, 25, 419.-3131 Santos, M. F. C. ; Dutra, L. M. ; Moraes, V. R. S. ; Barison, A. ; Costa, E. V. ; Biochem. Syst. Ecol.
2015, 60, 106.
Fraction III showed to be composed of three substances as indicated by HPLC retention times of 19.9, 22.4 and 25.4 min (Figure S12, SI section). In its 1H NMR spectrum, duplicated signals were also observed, suggesting the presence of two alkaloids of analogous structures, being a set of signals similar to that observed in fraction II (assigned to compound 5). In this way signals at δH 7.15/7.21 (H-3), and doublets at δH 7.80/7.99 (H-4) and 8.92/9.04 (H-5) were observed, with integration for one hydrogen each and can be attributed to the hydrogens a and b of the pyridine ring (Figure S11, SI section), characteristic of alkaloids with an aromatic ring B. 3030 Rabêlo, S. V. ; Costa, E. V. ; Barison, A. ; Dutra, L. M. ; Nunes, X. P. ; Tomaz, J. C. ; Rev. Bras. Farmacogn.
2015, 25, 419.,3131 Santos, M. F. C. ; Dutra, L. M. ; Moraes, V. R. S. ; Barison, A. ; Costa, E. V. ; Biochem. Syst. Ecol.
2015, 60, 106. Additionally, multiplets were also observed at δH 3.10 and 3.20 which may refer to signals H-4 and H5 of non-aromatic ring B from another alkaloid. These signals combined with others observed in δH 2.75 (N-CH3), and δH 7.04 (H-7) suggest the presence of a dehydroaporphine alkaloid. 3232 Sari, A. ; Gray, A. I. ; Sariyar, G. ; Nat. Prod. Res.
2004, 18, 265. Other signals were observed in the region of aromatic hydrogens such as doublets at δH 7.33 (H-10); 8.56 (H-11) and a singlet at δH 8.04 (H-8) as well as at δH 6.42 (-OCH2O) and comparative analyzes with data from literature suggest an aporphine alkaloid trisubstituted in ring D. 3030 Rabêlo, S. V. ; Costa, E. V. ; Barison, A. ; Dutra, L. M. ; Nunes, X. P. ; Tomaz, J. C. ; Rev. Bras. Farmacogn.
2015, 25, 419. The others signals were at δH 8.02/8.33 (H-8) and 7.52/8.75 (H-11) of singlets with integration for one hydrogen each referring to the two tetrasubstituted alkaloids in ring D. Additionally, the signals at δH 4.09 (OMe-C9) and 4.14 (OMe-C10), with integration for three hydrogens each, confirm the existence of the tetrassubstituted benzene ring (ring D) for two of the alkaloids (Figure S11, SI section). Comparison of NMR data with literature suggests the presence of oxoaporphine alkaloids 5 and 7 and one dehydroaporphin alkaloid 8 as constituents of fraction III. 3030 Rabêlo, S. V. ; Costa, E. V. ; Barison, A. ; Dutra, L. M. ; Nunes, X. P. ; Tomaz, J. C. ; Rev. Bras. Farmacogn.
2015, 25, 419.
31 Santos, M. F. C. ; Dutra, L. M. ; Moraes, V. R. S. ; Barison, A. ; Costa, E. V. ; Biochem. Syst. Ecol.
2015, 60, 106.
32 Sari, A. ; Gray, A. I. ; Sariyar, G. ; Nat. Prod. Res.
2004, 18, 265.-3333 Hufford, C. D. ; Funderburk, M. J. ; Morgan, J. M. ; Robertson, L. W. ; J. Nat. Prod.
1975, 64, 789.
The 1H NMR spectra of fraction IV (Figure S17, SI section), whose HPLC chromatogram analysis suggested the presence of three compounds Figure S18, SI section, was very similar to the spectrum of fraction I, composed exclusively of glaucine, suggesting presence of analogous alkaloids. Singlets were observed at the range of δH 6.64-6.97 (H-3) and multiplets between δH 2.88-3.00 (H-4 and H-5, H-7), besides signals at δH 4.05 (H-6a) that show the existence of non-aromatics rings B and C. The several methoxyl groups at δH 3.66-3.91, corroborate the existence of a benzene ring tetrasubstituted (ring D) for the three compounds but with different substitution pattern for one of them. Singlets at δH 6.76/6.80 (H-11) and 8.07/8.10 (H-8) evidenced the D ring substitution pattern for two of them,(34,35 whereas the presence of two doublets in 6.82 and 6.87 revealed that one of the alkaloids has a substitution pattern that locates the remaining hydrogens in the ortho position as in the case of isocorydine. 3636 Santos, M. F. C. ; Fontes, J. E. N. ; Dutra, L. M. ; Bomfim, L. M. ; Costa, C. O. D. ; Moraes, V. R. S. ; Barison, A. ; Soares, M. B. P. ; da Silva, F. M. A. ; Almeida, J. R. G. S. ; Koolen, H. H. F. ; Bezerra, D. P. ; Costa, E. V. ; Quim. Nova
2018, 41, 884. Additionally, the presence of two singlets at δH 2.64 (NMe) proves the existence of a methyl group linked to nitrogen for two of them and suggests that in one of the alkaloids there is no N substitution (Figure S17, SI section), in the case of norglaucine. 3434 Castro-Saavedra, S. ; Fuentes-Barros, G. ; Tirapegui, C. ; Acevedo-Fuentes, W. ; Cassels, B. K. ; Barriga, A. ; Vilches-Herrera, M. ; J. Chil. Chem. Soc.
2016, 61, 3076. Comparison with literature data suggested the presence of alkaloids 2,3 and 4. 3434 Castro-Saavedra, S. ; Fuentes-Barros, G. ; Tirapegui, C. ; Acevedo-Fuentes, W. ; Cassels, B. K. ; Barriga, A. ; Vilches-Herrera, M. ; J. Chil. Chem. Soc.
2016, 61, 3076.
35 Blanco, O. M. ; Castedo, L. ; Villaverde, M. C. ; Phytochemistry
2013, 32, 1055.-3636 Santos, M. F. C. ; Fontes, J. E. N. ; Dutra, L. M. ; Bomfim, L. M. ; Costa, C. O. D. ; Moraes, V. R. S. ; Barison, A. ; Soares, M. B. P. ; da Silva, F. M. A. ; Almeida, J. R. G. S. ; Koolen, H. H. F. ; Bezerra, D. P. ; Costa, E. V. ; Quim. Nova
2018, 41, 884.
UHPLC-HR-ESI-MS/MS analysis
All UHPLC-HR-ESI-MS in positive mode spectra showed [M + H]+ ions as the main peak. The most diagnostic fragment ions, [M + H - 17]+ or [M + H - 31]+ are commonly observed for aporphine alkaloids depending on the nature of substituent of the amino group (-NH2 or -N-CH3) (Figure 2). These losses come from key fragments of aporphine alkaloids. 3737 Soares, E. R. ; Silva, F. M. ; Almeida, R. A. ; Lima, B. R. ; Silva-Filho, F. A. ; Barison, A. ; Phytochem. Anal. 2015, 26, 339. In order to characterize aporphine alkaloids in the D. lanceolata extract, MS/MS experiment was performed to obtain more chemical structural information about the alkaloids produced by the species, allowing to distinguish the different types of aporphine alkaloids present in the plant’s leaves. Thus, depending on substitution, pattern losses of m/z 15 (Me); 31 (OMe), 28 (CO) or 32 (MeOH) can be observed. 1313 Fabre, N. ; Claparols, C. ; Richelme, S. ; Angelin, M. L. ; Fourasté, I. ; Moulis, C. ; J. Chromatogr. A 2000, 35, 904.
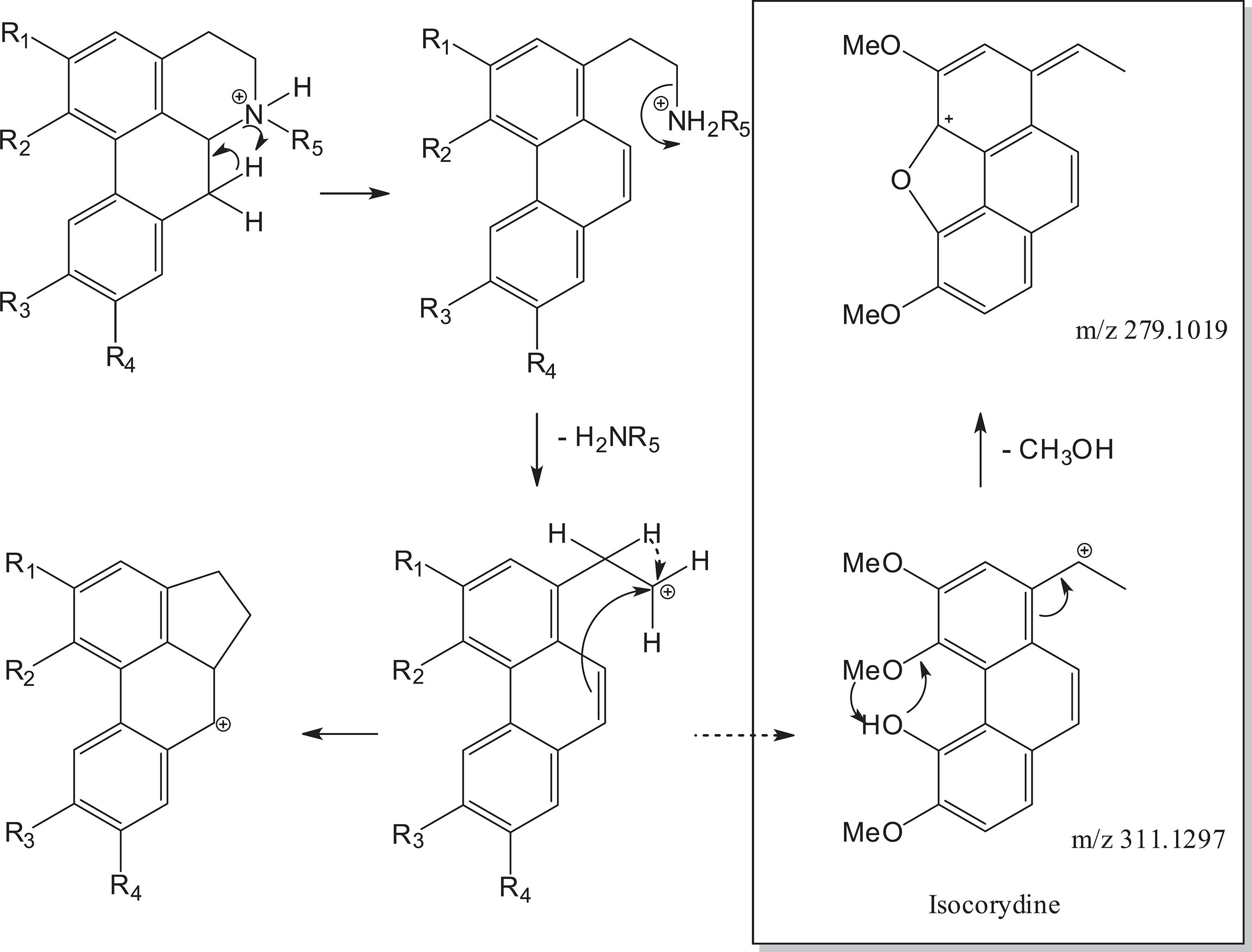
Proposed fragmentation mechanism for aporphine alkaloids 1-4. In box additional fragmentation of isocorydine (3).
For glaucine (1), present in fraction I, in addition to the loss of NH2CH3 (m/z 31.0419), the fragment at m/z 294.1237 is relative to the loss of the OCH3 radical (m/z 31.0183) and the fragment at m/z 310.1186 corresponds to a loss of CH3 radical from fragment m/z 325.1433 (Table 1). The loss of radical methyl or methoxy groups seem to be favored by the presence of an aromatic ring with one or more non-vicinal methoxy groups to an OH or one which does not have any OH. 1414 Stévigny, C. ; Jiwan, J. L. H. ; Rozenberg, R. ; Hoffmann, E. ; Quetin-Leclercq, J. ; Rapid Commun. Mass Spectrom. 2004, 18, 523. This is also observed for norglaucine (2) whose MS/MS spectra showed a peak at m/z 325.1427 corresponding to [M + H - NH3]+, confirming loss of NH3 (m/z 17.0265). Isocorydine (3), in turn, contains the hydroxy and a methoxy group in the vicinal positions, so this alkaloid has characteristic losses of CH3OH followed by CO. 1414 Stévigny, C. ; Jiwan, J. L. H. ; Rozenberg, R. ; Hoffmann, E. ; Quetin-Leclercq, J. ; Rapid Commun. Mass Spectrom. 2004, 18, 523. In UHPLC-HR-ESI-MS, fragments at m/z 296.1031 [M + H - CH3]+; 279.1019 [M + H - MeOH]+ were observed. The proposed fragmentation for alkaloids 1-4 is shown in Figure 2. Additional fragmentations of 3 are in the box of Figure 2.
Elemental composition of aphophine alkaloids (1-8) observed in UPHLC-ESI-HRMS ([M + H]+) and MS/MS spectra from D. lanceolata fractions; calculated exact mass (calcd.) and measured accurate mass (acc.) for each indicated precursor and product ion
Although 2,3 and 4 are isomers (molecular formula C20H24NO4) with [M + H]+ at m/z 342.1687, the fragmentation pattern is different, since norglaucine (2) fragmentation leads to loss of NH3 (m/z 17.0265) generating the product ion m/z 325.1427, observed in the MS/MS spectrum, while isocorydine (3) and N-methyllaurotetanine (4) fragmentation afforded CH3NH2 neutral loss corresponding to m/z 311.1267. Additionally, loss of CH3 radical gives rise to m/z 296.1060 for 3 and 4 (Figure 2). Distinction between 3 and 4 can be verified by the fragment obtained from loss of methoxyl group at m/z 280.1093 from fragment m/z 311.1267 (Table 1) to 4,1717 Nikolić, D. ; Gödecke, T. ; Chen, S. -N. ; White, J. ; Lankin, D. C. ; Pauli, G. F. ; van Breemen, R. B. ; Fitoterapia 2012, 83, 441. while for 3 there is the loss of a molecule of methanol that is only possible by the position of the hydroxyl group (Figure 2).
In fraction II it was possible to identify two oxoaporphine alkaloids: oxoglaucine ([M + H]+ at m/z 352.1180) and liriodenine ([M + H]+ at m/z 276.0654). These compounds, being oxoaporphines, do not allow the loss of the amino group, since the nitrogen atom is part of an aromatic nucleus. 1616 Pohlit, A. M. ; Oliveira, C. M. X. ; Lopes, J. L. C. ; Andreo, M. A. ; Niehues, M. ; Lopes, N. P. ; In Revisões em Processos e Técnicas Avançadas de Isolamento e Determinação de Ativos de Plantas Medicinais, vol. 1, 1st ed. ; Souza, G. H. B. ; Mello, J. C. P. ; Lopes, N. P. UFOP: Ouro Preto, 2012, p. 171. Thus, the fragmentations observed in the MS/MS spectra for these alkaloids correspond to the loss of methyl substituents of the methoxyl groups or loss of CO from a methylenedioxy group. Liriodenine (6) (C17H19NO3) is an alkaloid containing one methylenedioxy group and the fragmentation of this alkaloid showed a fragment at m/z 248.0705 for the [M + H - CO]+ ion,1616 Pohlit, A. M. ; Oliveira, C. M. X. ; Lopes, J. L. C. ; Andreo, M. A. ; Niehues, M. ; Lopes, N. P. ; In Revisões em Processos e Técnicas Avançadas de Isolamento e Determinação de Ativos de Plantas Medicinais, vol. 1, 1st ed. ; Souza, G. H. B. ; Mello, J. C. P. ; Lopes, N. P. UFOP: Ouro Preto, 2012, p. 171. while oxoglaucine (5) showed in its MS/MS spectrum the fragment [M + H - CH3]+ at m/z 337.0943 and at m/z 306.0762 corresponding to loss of an additional methoxyl radical. 3838 da Silva, F. M. A. ; Koolen, H. H. F. ; de Almeida, R. A. ; de Souza, A. D. L. ; Pinheiro, M. L. B. ; Costa, E. V. ; Quim. Nova 2012, 35, 944. Another oxoaporphine alkaloid, identified in fraction IV, lanuginosine (C18H12NO4) showed a [M + H]+ at m/z 306.0769 (calcd. 306.0766) containing, besides a methylenedioxy and a methoxy group, a peak at m/z 291.0522 in the MS/MS spectrum, corresponding to loss of a CH3 radical and from this another peak at m/z 263.0576 corresponding to loss of CO of one methylenedioxy group (Table 1). Dehydroglaucine (8) (present in fraction III) like oxoaporphine alkaloids does not allow the loss of amino group, due to the lack of one of the hydrogens in position 7. Thus, for 8, only loss of methyl radicals was observed in MS/MS spectrum including peak at m/z 339.1472 and 324.1236, corresponding to multiple loss of methyl radicals.
The literature reports chemical analysis of approximately 16 Duguetia species, resulting in the characterization of more than 100 different alkaloids. 44 Pérez, E. G. ; Cassels, B. K. In The Alkaloids: Chemistry and Biology, vol. 68, 1st ed. ; Cordell, G. A. , ed. ; Academic Press: London, 2010, p.83. Despite of this information, in the present work eight related aporphine alkaloids were identified, with 1,5 and 8 being described for the first time in Duguetia genus while the occurrence of alkaloids 2,3,4,6 and 7 have been described for the first time in D. lanceolata.
Antiparasitic activity
Fractions containing alkaloids were incubated with T. cruzi trypomastigotes and promastigotes of Leishmania (L. ) infantum and the viability detected by colorimetric assays. The most promising activity was observed for fraction I, constituted by glaucine. Additionally, this fraction demonstrated activity against the clinically relevant forms of L. infantum intracellular amastigotes, with a half maximal inhibitory concentration (IC50) value of 7.5 µg mL-1 (Table 2). Furthermore, glaucine also showed anti trypanosomal activity against intracellular amastigotes of T. cruzi and resulted in an IC50 value of 28.6 µg mL-1. No mammalian cytotoxicity was observed up to 70.0 µg mL-1, confirming the potential of glaucine to be used as a future scaffold for the design of new antiprotozoan derivatives. Fraction IV, constituted by norglaucine (2), isocorydine (3) and N-methyllaurotetanine (4), showed an IC50 value of 50.0 µg mL-1 against promastigotes of L. infantum, but no activity was detected against T. cruzi trypomastigotes (Table 2). Fraction II, constituted by oxoglaucine (5) and liriodenine (6), showed an IC50 value of 83.0 µg mL-1 against T. cruzi trypomastigotes (Table 2). Glaucine has been also described showing activity against epimastigote forms of Trypanosoma cruzi with an IC50 value of 90 µM. 3939 Morello, A. ; Lipchenca, I. ; Cassels, B. K. ; Speisky, H. ; Aldunate, J. ; Repetto, Y. ; Comp. Biochem. Physiol. , Part C: Pharmacol. , Toxicol. Endocrinol. 1994, 107, 367. Previous studies reported the antileishmanial and antitrypanosomal activity of crude extracts from isoquinoline alkaloid-producing families, including D. lanceolata,4040 Tempone, A. G. ; Borborema, S. E. T. ; Andrade, H. F. J. ; Amorim Gualda, N. C. ; Yogi, A. ; Carvalho, C. S. ; Bachiega, D. ; Lupo, F. N. ; Bonotto, S. V. ; Fischer, D. C. H. ; Phytomedicine 2005, 12, 382. but no information about the activity of the identified compounds have been described.
Alkaloidal fractions I-IV from D. lanceolata were assayed against L. infantum (promastigotes and amastigotes) and against T. cruzi (tripomastigotes and amastigotes) and expressed as effective concentration 50% (IC50) for parasites
Chagas disease and leishmaniasis affect more than 20 million people in developing countries. 4141 Coura, J. R. ; Mem. Inst. Oswaldo Cruz 2015, 110, 277.,4242 Burza, S. ; Croft, S. L. ; Boelaert, M. ; Lancet 2018, 392, 951. Considering the limited efficacy of the current therapies and highly toxic drugs, new safe and affordable treatments are needed. Our work investigated for the first time in literature the in vitro antitrypanosomal and antileishmanial efficacy of the aporphine alkaloid glaucine and other fractions against clinically relevant intracellular forms of the parasites and also demonstrate the potential of D. lanceolata in the search for bioactive compounds against neglected diseases.
Conclusions
The approach used for the dereplication of the alkaloid fraction extracted from the leaves of D. lanceolata assisted in the identification of four aporphine alkaloids, one derivative as well as three oxoaporphine alkaloids. Additionally, glaucine showed activity against the clinical forms of the protozoan intracellular amastigotes of parasites Leishmania infantum and Trypanosoma cruzi and no mammalian cytotoxicity and fraction IV constituted by isocorydine (3), norglaucine (2) and N-methyllaurotetanine (4) showed activity against promastigotes of Leishmania infantum. This data demonstrates the potential of aporphine alkaloids from D. lanceolata St. -Hil (Annonaceae) in the search for new drug candidates for neglected diseases.
-
Supplementary InformationSupplementary data are available free of charge at http://jbcs.sbq.org.br as PDF file.
Acknowledgments
The authors thank FAPESP for financial support for the development of this work (2016/24985-4 and 2018/10279-6). We also thank CAPES fellowships to EPD and JMC (CAPES Cod. 001), and CNPq for the scientific research awards of PS, AGT and JHGL.
References
-
1Chatrou, L. W. ; Rainer, H. ; Maas, P. J. M. In Flowering Plants of the Neotropics; Smith, N. ; Mori, S. A. ; Henderson, A. ; Stevenson, D. W. ; Heald, S. V. , eds. ; The New York Botanical Garden: New York, 2004, p. 18.
-
2Ribeiro, J. E. L. S. ; Hopkins, M. J. G. ; Vicentini, A. ; Sothers, C. A. ; Costa, M. A. S. ; Brito, J. M. ; Souza, M. A. D. ; Martins, L. H. P. ; Lohmann, L. G. ; Assunção, P. A. C. L. ; Pereira, E. C. ; Silva, C. F. ; Mesquita, M. R. ; Procópio, L. C. ; Flora da Reserva Ducke: Guia de Identificação das Plantas Vasculares de uma Floresta de Terra Firme na Amazônia Central; INPA, DFID: Manaus, 1999.
-
3Leboeuf, M. ; Cavé, A. ; Bhaumix, P. K. ; Mukherjee, B. ; Mukherjee, R. ; Phytochemistry 1982, 21, 2783.
-
4Pérez, E. G. ; Cassels, B. K. In The Alkaloids: Chemistry and Biology, vol. 68, 1st ed. ; Cordell, G. A. , ed. ; Academic Press: London, 2010, p.83.
-
5Lúcio, A. S. S. C. ; Almeida, J. R. G. S. ; da-Cunha, E.V. L. ; Tavares, J. F. ; Barbosa Filho, J. M. ; In The Alkaloids: Chemistry and Biology, vol. 74, 1st ed. ; Knölker, H. -J. , ed. ; Academic Press: London, 2015, p. 233.
-
6Cavé, A. ; Leboeuf, M. ; Waterman, P. G. In Alkaloids: Chemical and Biological Perspectives, vol. 5, 1st ed. ; Pelletier, S. W. , ed. ; John Wiley: New York, USA, 1987, p. 133.
-
7Isernhagen, I. ; Listagem Florística de Espécies Arbóreas e Arbustivas de Mato Grosso: Um Ponto de Partida para Projetos de Restauração Ecológica, vol. 1, 1st ed. ; Embrapa Agrossilvipastoril: Sinop, 2015.
-
8Maas, P. J. ; de Kamer, H. M. V. ; Junikka, L. ; de Mello-Silva, R. ; Rainer, H. ; Rodriguésia 2001, 52, 61.
-
9Alves, D. S. ; Morejón, R. C. ; Machado, A. R. T. ; Carvalho, G. A. ; Pina, O. ; Oliveira, D. F. ; Semina: Ciênc. Agrár. 2015, 36, 4119.
-
10Gonçalves, G. L. P. ; Domingues, V. C. ; Ribeiro, L. P. ; Fernandes, J. B. ; Fernandes, M. F. G. ; Forim, M. R. ; Vendramim, J. D. ; Ind. Crops Prod 2017, 97, 360.
-
11Sousa, O. V. ; Soares Jr. , D. T. ; Del-Vechio, G. ; Mattosinhos, R. G. ; Gattass, C. R. ; Kaplan, M. A. C. ; Rev. Bras. Farmacogn 2004, 14, 11.
-
12Sousa, O. V. ; Del-Vechio-Vieira, G. ; Alves, M. S. ; Araújo, A. A. L. ; Pinto, M. A. O. ; Amaral, M. P. H. ; Rodarte, M. P. ; Kaplan, M. A. C. ; Molecules 2012, 17, 11056.
-
13Fabre, N. ; Claparols, C. ; Richelme, S. ; Angelin, M. L. ; Fourasté, I. ; Moulis, C. ; J. Chromatogr. A 2000, 35, 904.
-
14Stévigny, C. ; Jiwan, J. L. H. ; Rozenberg, R. ; Hoffmann, E. ; Quetin-Leclercq, J. ; Rapid Commun. Mass Spectrom. 2004, 18, 523.
-
15Carnevale Neto, F. ; Andréo, M. A. ; Raftery, D. ; Lopes, J. L. C. ; Lopes, N. P. ; Castro-Gamboa, I. ; Maia, B. H. L. N. S. ; Costa, E. V. ; Vessecchi, R. ; Rapid Commun. Mass Spectrom , in press DOI: 10.1002/rcm.8533.
» https://doi.org/10.1002/rcm.8533 -
16Pohlit, A. M. ; Oliveira, C. M. X. ; Lopes, J. L. C. ; Andreo, M. A. ; Niehues, M. ; Lopes, N. P. ; In Revisões em Processos e Técnicas Avançadas de Isolamento e Determinação de Ativos de Plantas Medicinais, vol. 1, 1st ed. ; Souza, G. H. B. ; Mello, J. C. P. ; Lopes, N. P. UFOP: Ouro Preto, 2012, p. 171.
-
17Nikolić, D. ; Gödecke, T. ; Chen, S. -N. ; White, J. ; Lankin, D. C. ; Pauli, G. F. ; van Breemen, R. B. ; Fitoterapia 2012, 83, 441.
-
18Sun, M. ; Liun, J. ; Lin, C. ; Miao, L. ; Lin, L. ; Acta Pharm. Sin. B 2014, 4, 208.
-
19Lima, B. R. ; da Silva, F. M. A. ; Soares, E. R. ; de Almeida, R. A. ; da Silva-Filho, F. A. ; Barison, A. ; Costa, E. V. ; Koolen, H. H. F. ; de Souza, A. D. L. ; Pinheiro, M. L. B. ; J. Braz. Chem. Soc. 2020, 31, 79.
-
20Tada, H. ; Shiho, O. ; Kuroshima, K. : Koyama, M. ; Tsukamoto, K. ; J. Immunol. Methods 1986, 93, 157.
-
21Pinto, E. G. ; Pimenta, D. C. ; Antoniazzi, M. M. ; Jared, C. ; Tempone, A. G. ; Exp. Parasitol 2013, 135, 655.
-
22Ferreira, D. D. ; Mesquita, J. T. ; Silva, T. A. C. ; Romanelli, M. M. ; Batista, D. G. J. ; da Silva, C. F. ; da Gama, A. N. F. ; Neves, B. J. ; Melo-Filho, C. C. ; Soeiro, M. N. C. ; Andrade, C. H. ; Tempone, A. G. ; J. Venomous Anim. Toxins Incl. Trop. Dis. 2018, 24, 30.
-
23Wulsten, I. F. ; Costa-Silva, T. A. ; Mesquita, J. T. ; Lima, M. L. ; Galuppo, M. K. ; Taniwaki, N. N. ; Borborema, S. ; Costa, F. B. ; Schmidt, T. J. ; Tempone, A. G. ; Molecules 2017, 22, 685.
-
24Carollo, C. A. ; Siqueira, J. M. ; Garcez, W. S. ; Diniz, R. ; Fernandes, N. G. ; Nat. Prod. 2006, 69, 1222.
-
25Huang, W. J. ; Chen, C. H. ; Singh, O. V. ; Lee, S. L. ; Lee, S. S. ; Synth. Commun. 2002, 32, 3681.
-
26Kan, S. T. ; Ahmad, M. S. ; Azziz, S. S. S. A. ; Awang, K. ; Mhd Bakri, Y. ; Nafiah, M. A. ; Abbas, P. ; Hashim, Y. Z. H. -Y. ; Res. Pharm 2019, 23, 217.
-
27Hsieh, T. J. ; Wu, Y. C. ; Chen, S. C. ; Huang, C. S. ; Chen, C. Y. ; J. Chin. Chem. Soc 2004, 51, 869.
-
28Wijeratne, E. M. K. ; Hatanaka, Y. ; Kikuchi, T. ; Tezuka Y. ; Gunatilaka, A. A. L. ; Phytochemistry 1996, 42, 1703.
-
29Harrigan, G. G. ; Gunatilaka, A. L. ; Kingston, D. G. ; Chan, G. W. ; Johnson, R. K. ; Nat. Prod 1994, 57, 68.
-
30Rabêlo, S. V. ; Costa, E. V. ; Barison, A. ; Dutra, L. M. ; Nunes, X. P. ; Tomaz, J. C. ; Rev. Bras. Farmacogn. 2015, 25, 419.
-
31Santos, M. F. C. ; Dutra, L. M. ; Moraes, V. R. S. ; Barison, A. ; Costa, E. V. ; Biochem. Syst. Ecol. 2015, 60, 106.
-
32Sari, A. ; Gray, A. I. ; Sariyar, G. ; Nat. Prod. Res. 2004, 18, 265.
-
33Hufford, C. D. ; Funderburk, M. J. ; Morgan, J. M. ; Robertson, L. W. ; J. Nat. Prod. 1975, 64, 789.
-
34Castro-Saavedra, S. ; Fuentes-Barros, G. ; Tirapegui, C. ; Acevedo-Fuentes, W. ; Cassels, B. K. ; Barriga, A. ; Vilches-Herrera, M. ; J. Chil. Chem. Soc. 2016, 61, 3076.
-
35Blanco, O. M. ; Castedo, L. ; Villaverde, M. C. ; Phytochemistry 2013, 32, 1055.
-
36Santos, M. F. C. ; Fontes, J. E. N. ; Dutra, L. M. ; Bomfim, L. M. ; Costa, C. O. D. ; Moraes, V. R. S. ; Barison, A. ; Soares, M. B. P. ; da Silva, F. M. A. ; Almeida, J. R. G. S. ; Koolen, H. H. F. ; Bezerra, D. P. ; Costa, E. V. ; Quim. Nova 2018, 41, 884.
-
37Soares, E. R. ; Silva, F. M. ; Almeida, R. A. ; Lima, B. R. ; Silva-Filho, F. A. ; Barison, A. ; Phytochem. Anal. 2015, 26, 339.
-
38da Silva, F. M. A. ; Koolen, H. H. F. ; de Almeida, R. A. ; de Souza, A. D. L. ; Pinheiro, M. L. B. ; Costa, E. V. ; Quim. Nova 2012, 35, 944.
-
39Morello, A. ; Lipchenca, I. ; Cassels, B. K. ; Speisky, H. ; Aldunate, J. ; Repetto, Y. ; Comp. Biochem. Physiol. , Part C: Pharmacol. , Toxicol. Endocrinol. 1994, 107, 367.
-
40Tempone, A. G. ; Borborema, S. E. T. ; Andrade, H. F. J. ; Amorim Gualda, N. C. ; Yogi, A. ; Carvalho, C. S. ; Bachiega, D. ; Lupo, F. N. ; Bonotto, S. V. ; Fischer, D. C. H. ; Phytomedicine 2005, 12, 382.
-
41Coura, J. R. ; Mem. Inst. Oswaldo Cruz 2015, 110, 277.
-
42Burza, S. ; Croft, S. L. ; Boelaert, M. ; Lancet 2018, 392, 951.
Publication Dates
-
Publication in this collection
19 Aug 2020 -
Date of issue
Sept 2020
History
-
Received
16 Sept 2019 -
Accepted
15 May 2020