OBJECTIVE:
To assess the efficacy and safety of sitagliptin compared with voglibose added to combined metformin and insulin in patients with newly diagnosed type 2 diabetes (T2DM).
METHODS:
In this 12-week prospective, randomized, parallel trial, 70 newly diagnosed T2DM patients with glycosylated hemoglobin (HbA1c) ≥9% and/or fasting plasma glucose (FPG) ≥11.1 mmol/L were randomized (1:1) to receive sitagliptin 100 mg per day + metformin + insulin glargine or voglibose 0.2 mg three times daily + metformin + insulin glargine. Change in HbA1c at week 12 was the primary endpoint.
RESULTS:
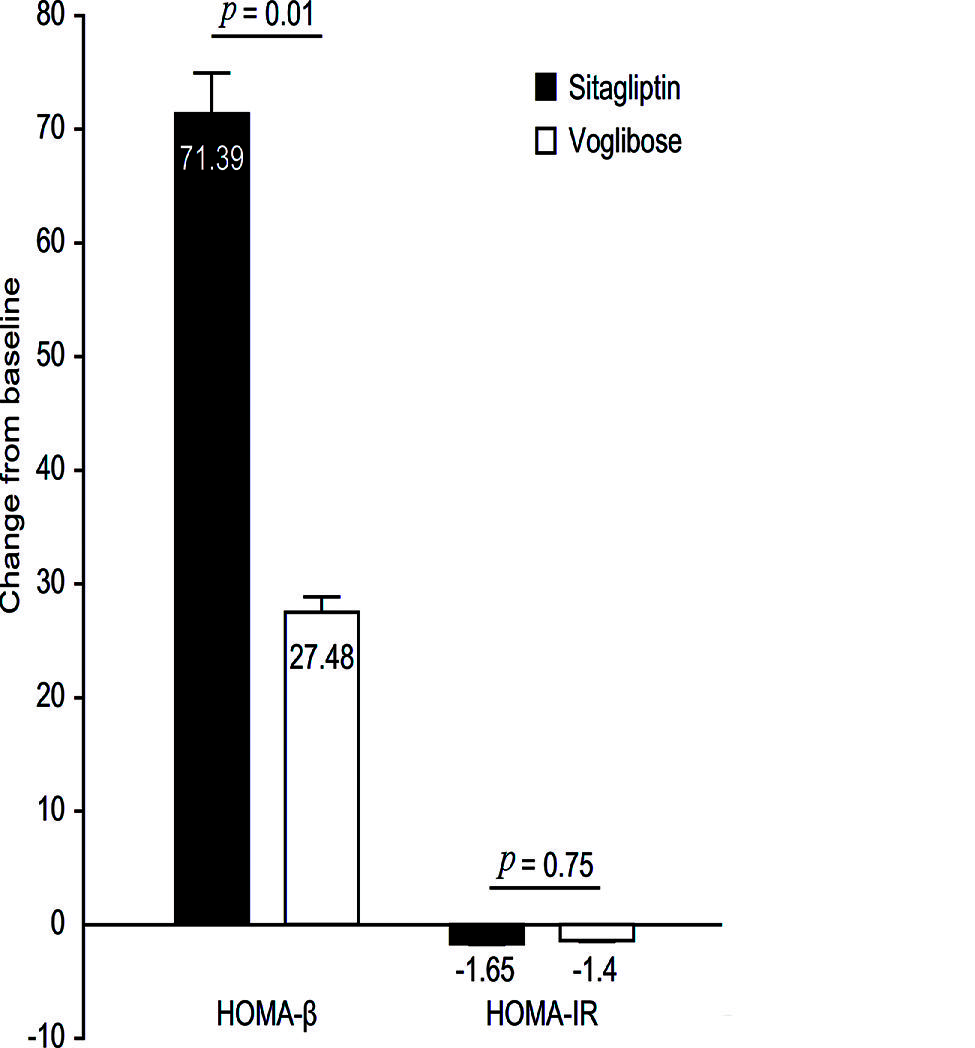
The mean baseline HbA1c was 11.0% in the patients. The changes in HbA1c from baseline were -6.00% in the sitagliptin group and -3.58% in the voglibose group, and the between-group difference was -2.42% (95% CI -1.91 to -2.93, p=0.02). The differences in FPG and homeostatic model assessment of β-cell function (HOMA-β) and the change in body weight between groups from baseline were -2.95 mmol/L (p=0.04), 43.91 (p=0.01) and -2.23 kg (p=0.01), respectively. One patient (2.9%) in the sitagliptin group and three patients (8.6%) in the voglibose group exhibited hypoglycemia.
CONCLUSIONS:
Sitagliptin added to combined metformin and insulin therapy showed greater efficacy and good safety regarding hypoglycemia in patients with newly diagnosed T2DM compared with voglibose.
Diabetes Mellitus, Type 2; Sitagliptin Phosphate; Voglibose; Combined Therapy; Efficacy; Safety