ABSTRACT
In order to investigate the existence of a possible abscission zone in the binding region between the peduncle and the coffee fruit, as well as the biochemical events involved in fruit ripening, we studied peduncles and fruits of Coffea arabica L. cv. “Icatu amarelo”. Coffee fruits were harvested at different maturation stages by using specific intervals of detachment force. It was observed a breakdown in the cells of the connecting region between peduncles and fruits throughout the maturation process. This disruption is probably caused by increased activity of the cell wall-loosening enzymes (polygalacturonase and cellulase) as well as by higher levels of lipid peroxidation in the final maturation stages. In general, physiological differences between maturation stages were observed and can largely explain the different detachment forces required at different stages. In conclusion, there is no clear abscission zone between the peduncle and the coffee fruit cv. “Icatu amarelo”. Nevertheless, the decrease in the detachment force throughout the maturation process can occur due to the activity of cell wall-degrading enzymes together with increased oxidative stress during the fruit ripening.
Key words
Coffea arabica; Icatu amarelo; mechanized harvesting; degrading enzyme; oxidative stress
INTRODUCTION
The operating speed and the vibration of the rods of the harvester during mechanized harvesting of coffee are determined by the force required to detach the fruit from the plant (Silva et al. 2010Silva, F. C., Silva, F. M., Alves, M. C., Barros, M. M. and Sales, R. S. (2010). Comportamento da força de desprendimento dos frutos de cafeeiros ao longo do período de colheita. Ciência e Agrotecnologia, 34, 468-474. http://dx.doi.org/10.1590/S1413-70542010000200028.
http://dx.doi.org/10.1590/S1413-70542010...
; 2013Silva, F. C., Silva, F. M., Silva, A. C., Barros, M. M. and Palma, M. A. Z. (2013). Desempenho operacional da colheita mecanizada e seletiva do café em função da força de desprendimento dos frutos. Coffee Science, 8, 53-60.; 2014Silva, F. M., Silva, F. O. E. and Miranda, N. B. (2014). Mecanização da colheita viabiliza cafeicultor. Granja, 784, 60-62.). Thus, to maximize harvest efficiency, it is necessary to adjust the vibration of the rods to avoid the harvesting of green fruits, since there are variations of fruit detachment force (FDF) in green and cherry fruits among cultivars and maturation period (Silva et al. 2010Silva, F. C., Silva, F. M., Alves, M. C., Barros, M. M. and Sales, R. S. (2010). Comportamento da força de desprendimento dos frutos de cafeeiros ao longo do período de colheita. Ciência e Agrotecnologia, 34, 468-474. http://dx.doi.org/10.1590/S1413-70542010000200028.
http://dx.doi.org/10.1590/S1413-70542010...
). In recent years, a FDF from 2 to 5.9 N has been adopted as optimal for mechanized and selective harvesting of coffee (Silva et al. 2013Silva, F. C., Silva, F. M., Silva, A. C., Barros, M. M. and Palma, M. A. Z. (2013). Desempenho operacional da colheita mecanizada e seletiva do café em função da força de desprendimento dos frutos. Coffee Science, 8, 53-60.).
The abscission of leaves, flowers and fruits is a highly-regulated phenomenon involving structural, biochemical and molecular changes (Lewis et al. 2006Lewis, M. W., Leslie, M. E. and Liljegren, S. J. (2006). Plant separation: 50 ways to leave your mother. Current Opinion in Plant Biology, 9, 59-65. http://dx.doi.org/10.1016/j.pbi.2005.11.009.
http://dx.doi.org/10.1016/j.pbi.2005.11....
). Usually this process takes place in an anatomically distinct layer of cells known as the abscission zone (Estornell et al. 2013Estornell, L. H., Agustí, J., Merelo, P., Talón, M. and Tadeo, F. R. (2013). Elucidating mechanisms underlying organ abscission. Plant Science, 199-200, 48-60. http://dx.doi.org/10.1016/j.plantsci.2012.10.008.
http://dx.doi.org/10.1016/j.plantsci.201...
). Abscission begins with cell wall loosening within this zone due to the dissolution of the middle lamella, followed by complete enzymatic degradation of cell wall. Degradation is mediated by the action of enzymes, such as polygalacturonase (PG) and cellulase, and culminates in a loss of intercellular adhesion (Rodionova et al. 2001Rodionova, N. A., Dubovaya, N. V., Martinovich, L. I. and Bezborodov, A. M. (2001). Formation of extracellular enzyme systems during Geotrichum candidum 3C growing on the cell walls isolated from cereal grains. Applied Biochemistry and Microbiology, 37, 562-565. http://dx.doi.org/10.1023/B:RUPP.0000003277.22914.6c.
http://dx.doi.org/10.1023/B:RUPP.0000003...
). Reactive oxygen species (ROS) also play an important role in cell wall loosening. For example, exogenous application of H2O2 in Capsicum plants led to an increase in the expression of genes encoding the cellulase enzyme and resulted in leaf abscission (Sakamoto et al. 2008Sakamoto, M., Munemura, I., Tomita, R. and Kobayashi, K. (2008). Involvement of hydrogen peroxide in leaf abscission signaling, revealed by analysis with an in vitro abscission system in Capsicum plants. The Plant Journal, 58, 13-27. http://dx.doi.org/10.1111/j.1365-313X.2008.03577.x.
http://dx.doi.org/10.1111/j.1365-313X.20...
). The mechanisms of membrane deterioration and tissue disruption during ripening are similar to those observed during senescence (Mondal et al. 2009Mondal, K., Malhotra, S. P., Jain, V. and Singh, R. (2009). Oxidative stress and antioxidant systems in Guava (Psidium guajava L.) fruits during ripening. Physiology and Molecular Biology of Plants, 15, 327-334. http://dx.doi.org/10.1007/s12298-009-0037-3.
http://dx.doi.org/10.1007/s12298-009-003...
).
Although abscission zones have been recorded in the fruits of several species, as Olea europaea L. (Parra-Lobato and Gomez-Jimenez 2011Parra-Lobato, M. C. and Gomez-Jimenez, M. C. (2011). Polyamine-induced modulation of genes involved in ethylene biosynthesis and signalling pathways and nitric oxide production during olive mature fruit abscission. Journal of Experimental Botany, 62, 4447-4465. http://dx.doi.org/10.1093/jxb/err124.
http://dx.doi.org/10.1093/jxb/err124...
), there is still a lack of structural studies related to FDF in coffee fruits. In order to investigate the existence of a possible abscission zone in the binding region between the peduncle and the coffee fruit, as well as the biochemical events involved in fruit ripening, we studied peduncles and fruits of Coffea arabica L. cv. “Icatu amarelo”. The characterization of the abscission zone throughout the ripening process and its relationship with the detachment force applied by harvesters will significantly contribute to the improvement of selective mechanical harvesting of coffee.
MATERIALS AND METHODS
Fruits and peduncles of Coffea arabica L. cv. “Icatu amarelo” were collected individually at different stages of maturation and with different degrees of detachment force (Table 1). FDF was measured by a portable dynamometer Instrutemp (DD-200), adapted for coffee fruits. Detachment force intervals for each stage of fruit ripening were determined and fixed by a pre-test conducted at the place of the harvest, based on the results reported by Silva et al. (2013)Silva, F. C., Silva, F. M., Silva, A. C., Barros, M. M. and Palma, M. A. Z. (2013). Desempenho operacional da colheita mecanizada e seletiva do café em função da força de desprendimento dos frutos. Coffee Science, 8, 53-60.. Fruits were collected individually, and the region of connection between the peduncle and the fruit was excised with a blade (Figure 1a) or manually (Figure 1b).
Fruits ripening stage, harvesting period and detachment force intervals of fruits and peduncles of Coffea arabica cv. “Icatu amarelo”.
Schematic illustration of a coffee fruit with peduncle. This scheme shows the samples and their application for different storage methods before ltrastructural, anatomical and biochemical analyses (a). The grey circle (b) is a demonstration of the top view of sample “e”. Dashed lines point out the cuts of the blade.
Samples fixed (sample “a”) in modified Karnovsky (2.5% glutaraldehyde, 2.0% paraformaldehyde, 0.05 M cacodylate buffer, pH 7,2) were placed in glycerol 30% for 30 min and, subsequently, immersed in liquid nitrogen. A longitudinal section was then performed with a blade (Figure 1a). Peduncles stored in Karnovsky (Figure 1b) were excised in the proximal region of the fruit, and sample “e” was analyzed. The samples (“a” and “e”) were washed 3 times for 10 min in 0.05 M cacodylate buffer and post-fixed in 1% osmium tetroxide for 2 h. They were then dehydrated in gradient of acetone (25; 50; 75 and 90%) for 10 min each and twice in 100% acetone for 10 min and placed in critical point apparatus (Balzers CPD 030) using liquid CO2 to complete the drying. Samples were assembled on aluminum brackets (stubs), with double carbon tape, recovered by a gold layer using an evaporator (Balzers SCD 050) and kept on silica gel for observation. Specimens from sample “a” were arranged with the excised area “up”, whereas those from sample “e” were mounted with the fruit region “up”. Scanning electron microscope (SEM) analyses were performed using a Leo EVO 40, 5.5 to 9 mm working distance and 20 kV.
The region connecting the peduncle and the fruit was fixed in FAA (formaldehyde, ethanol and acetic acid) for 72 h and then preserved in 70% ethanol. For the permanent blades, samples were dehydrated in ethanol gradient, infiltrated and embedded in hydroxyethyl methacrylate Leica®. The sections were obtained using a semiautomatic microtome with a thickness of 8 microns. The sections were extended on the blades containing water, and, when dried, the material was stained with toluidine blue (O’Brien et al. 1964O’Brien, T. P., Feder, N. and McCully, M. E. (1964). Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma, 59, 368-373. http://dx.doi.org/10.1007/BF01248568.
http://dx.doi.org/10.1007/BF01248568...
). The blades were photographed with a Zeiss Axio Cam Erc 5s in a camera attached to a Zeiss Axio model Lab.A1 microscope.
Samples stored at − 80 °C (samples “c” and “d”) were used for the biochemical analyses. Sample “c” contained intact peduncle and a small portion of the fruit. Samples “d” contained only the fruit without the peduncle.
PG activity was performed with the Buescher and Furmanski method (1978)Buescher, R. W. and Furmanski, R. J. (1978). Role of pectinesterase and polygalacturonase in the formation of woolliness in peaches. Journal of Food Science, 43, 264-266. http://dx.doi.org/10.1111/j.1365-2621.1978.tb09788.x.
http://dx.doi.org/10.1111/j.1365-2621.19...
, modified by Vilas-Boas et al. (1996)Vilas-Boas, E. V. B., Chitarra, A. B. and Chitarra, M. I. F. (1996). Modificações pós-colheita de bananas ‘Prata’ y-irradiada. Pesquisa Agropecuária Brasileira, 31, 599-607.. The extract was obtained by maceration of 1.5 g of fresh weight in liquid nitrogen and subsequent addition of 5 mL of 1 M NaCl, followed by addition of 1 mL of 0.25% citrus pectin and immersion in a water bath at 30 °C for 3 h. The reaction was stopped by boiling for 5 min, after which the samples were deproteinized with 0.3 N barium hydroxide, 5% zinc sulfate and water. The samples were filtered and used for the determination of enzymatic activity by Somoghi-Nelson’s method (Nelson 1944Nelson, N. A. (1944). A photometric adaptation of Somogyi method for the determination of glucose. The Journal of Biological Chemistry, 153, 375-380.). Results were expressed as ῃmol of galacturonic acid per g of fresh material per min.
Cellulase activity was determined according to Walker et al. (2006)Walker, D., Ledesma, P., Delgado, O. D. and Breccia, J. D. (2006). High endo β-1,4-D-glucanase activity in a broad pH range from the alkali-tolerant Nocardipsis sp. SES28. World Journal of Microbiology and Biotechnology, 22, 761-764. http://dx.doi.org/10.1007/s11274-005-9097-x.
http://dx.doi.org/10.1007/s11274-005-909...
with modifications. Enzymatic extract was obtained by macerating 0.3 g of fresh weight in liquid nitrogen to which 1.5 mL of 1 M NaCl was added. The solution was centrifuged at 12,000 g for 30 min at 4 °C, and the supernatant was collected for analysis of enzymatic activity; 400 µL of the extract were added to 400 µL of 1% carboxymethylcellulose and incubated in a water bath at 30 °C for 1 h. Deproteinization was performed by adding 3 N barium hydroxide, 5% zinc sulfate and water to the samples, followed by centrifugation at 8,000 g for 20 min at 25 °C. Enzymatic activity was determined by the DNS method (Miller 1959Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426-428. http://dx.doi.org/10.1021/ac60147a030.
http://dx.doi.org/10.1021/ac60147a030...
): aliquots of the samples were added to 400 µL of DNS and were boiled for 10 min. The absorbance of the samples was determined at 540 nm. One cellulase unit is defined as the amount of enzyme required to break one 1 µmol of reducing sugars per hour.
Hydrogen peroxide (H2O2) was determined according to Velikova et al. (2000)Velikova, V., Yordanov, I. and Edreva, A. (2000). Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Science, 151, 59-66. http://dx.doi.org/10.1016/S0168-9452(99)00197-1.
http://dx.doi.org/10.1016/S0168-9452(99)...
and lipid peroxidation, by the quantification of thiobarbituric acid reactive species, as described by Buege and Aust (1978)Buege, J. A. and Aust, S. D. (1978). Microsomal lipid peroxidation. Methods in Enzymology, 52, 302-310. http://dx.doi.org/10.1016/S0076-6879(78)52032-6.
http://dx.doi.org/10.1016/S0076-6879(78)...
.
Fruits and peduncles were collected in the 3rd middle of the coffee plants and each maturation stage and FDF range contained 4 replicates. Data were subjected to analysis of variance using the statistical program Sisvar 4.3 (System Analysis of Variance for Balanced Data) (Ferreira 2011Ferreira, D. F. (2011). Sisvar: a computer statistical analysis system. Ciência e Agrotecnologia, 35, 1039-1042. http://dx.doi.org/10.1590/S1413-70542011000600001.
http://dx.doi.org/10.1590/S1413-70542011...
). Means between treatments were compared by the Scott and Knott (1974)Scott, A. J., Knott, M. (1974). A cluster analysis method for grouping means in the analysis of variance. Biometrics, 30, 507-512. http://dx.doi.org/10.2307/2529204.
http://dx.doi.org/10.2307/2529204...
test (p ≤ 0.05).
RESULTS AND DISCUSSION
In general, there were no observable differences in cell structure between fruits at different stages, as well as between the forces used. The abscission zone was not detected (Figure 2).
Scanning electron micrographs of the connection region between peduncles and fruits. (a) Green stage with detachment force of 10 to 13 N; (b) The center of the cell and the periphery; (c) Green stage with detachment force of 8 to 9.9 N; (d) The center of the cell and the periphery; (e) Green-yellow stage with detachment force of 7 to 7.9 N; (f) The center of the cell and the periphery; (g) Green-yellow stage with detachment force of 6 to 6.9 N; (h) The center of the cells and the periphery; (i) Cherry stage with detachment force of 4 to 5.9 N; (j) The center of the cells and the periphery; (k) Cherry stage with detachment force of 2 to 3.9 N; (l) The center of the cells and the periphery. Scale bar: 200 μm in (a); (c); (e); (g); (i) and (k) and 100 μm in (b); (d); (f); (h); (j) and (l) (sample “a”).
Peduncles that were manually detached from the fruits (sample “e”) (Figure 3) at the green stage, regardless of the detachment force used, had disrupted cell walls in the central and peripheral regions. Disruption was more organized in the green-yellow and cherry stages, with cells arranged in the central portion and in the connecting region between fruits and peduncles.
Scanning electron micrographs of binding region between peduncles and fruits. (a) Green stage with detachment force of 10 to 13 N; (b) The center of the cell; (c) Periphery; (d) Green stage with detachment force of 8 to 9.9 N; (e) The center of the cell; (f) Periphery; (g) Green-yellow stage with detachment force of 7 to 7.9 N; (h) The center of the cell; (i) Periphery; (j) Green-yellow stage with detachment force of 6 to 6.9 N; (k) The center of the cells; (l) Periphery; (m) Cherry stage with detachment force of 4 to 5.9 N; (n) The center of the cells; (o) Periphery; (p) Cherry stage with detachment force of 2 to 3.9 N; (q) The center of the cells; (r) Periphery. Scale bar: 200 μm in (a); (d); (g); (j); (m) and (p) and 100 μm in (b); (c); (e); (f); (h); (i); (k); (l); (n); (o); (q) and (r) (sample “e”). Square means the observed areas in the center and the periphery; arrows indicate the cells.
In the green stage (Figure 3a to f), the peduncle was strongly attached to the fruit, requiring higher force for detachment (10 to 13 and 8 to 9.9 N). The manual detachment of the peduncle causes cell disruption and, consequently, the cells were more disorganized. In green-yellow fruits (Figure 3g to l), there was a disruption and a slight organization of cells, without apparent breakdown of the cell wall. This can be explained by the decrease in fruit detachment force (7 to 7.9 and 6 to 6.9 N) and the physiological changes associated with the beginning of maturation. In contrast, in the cherry stage (Figure 3m,r), the fruits were mature, and the cells had higher degree of loosening. This explains the higher organization of cells and the smaller force required to harvest (4 to 5.9 and 2 to 3.9 N). In this case, the cells were naturally disorganized and did not show any signs of forced breaking.
In summary, although there was a reduction in the FDF during maturation due to cell loosening, there was no evidence of an abscission zone (cells showing disruption without a linear pattern). Abscission zone cells, when present, are characterized by dense cytoplasm, interconnecting plasmodesmata and by a variable number among species (Estornell et al. 2013Estornell, L. H., Agustí, J., Merelo, P., Talón, M. and Tadeo, F. R. (2013). Elucidating mechanisms underlying organ abscission. Plant Science, 199-200, 48-60. http://dx.doi.org/10.1016/j.plantsci.2012.10.008.
http://dx.doi.org/10.1016/j.plantsci.201...
).
As in the SEM analysis, the anatomical one provided no evidence of a breakdown of the cells in a specific linear zone of the peduncle in any of the maturation stages and detachment forces (Figure 4). There was only a separation of some cells in the final maturation stage (Figure 3n,o), probably due to the action of cell wall-degrading enzymes. These results indicate that coffee fruits detach from the branches by the loosening of some cells in the connecting region between peduncle and fruit instead of forming an abscission layer. Thus, the fruit ripens on the branch, changing its color as it progresses through characteristic developmental stages. Indeed, in coffee crops, it is common to have fruits at different maturation stages on the same branches. It is believed that dried fruit drops primarily as a result of mechanical disruption (by dehydration) of the peduncle tissues. Thus, under natural conditions, fruit fall is probably due to external factors such as wind and rain (Chaves Filho 2007Chaves Filho, J. T. (2007). Novos paradigmas na fisiologia do cafeeiro. Documentos- IAC, 80, 67-74.).
Photomicrographs of anatomical sections of the proximal binding region between peduncles and fruits (sample “b”). (a) to (c) Green stage, with detachment force of 10 to 13 N; (d) to (f) Green stage with detachment force of 8 to 9.9 N; (g) – (i) Green-yellow stage with detachment force of 7 to 7.9 N; (j) – (l) Green-yellow stage with detachment force of 6 to 6.9 N; (m) – (o) Cherry stage with detachment force of 4 to 5.9 N; (p) – (r) Cherry stage with detachment force of 2 to 3.9 N. Scale bar: 10 μm. Arrows indicate cell disruption.
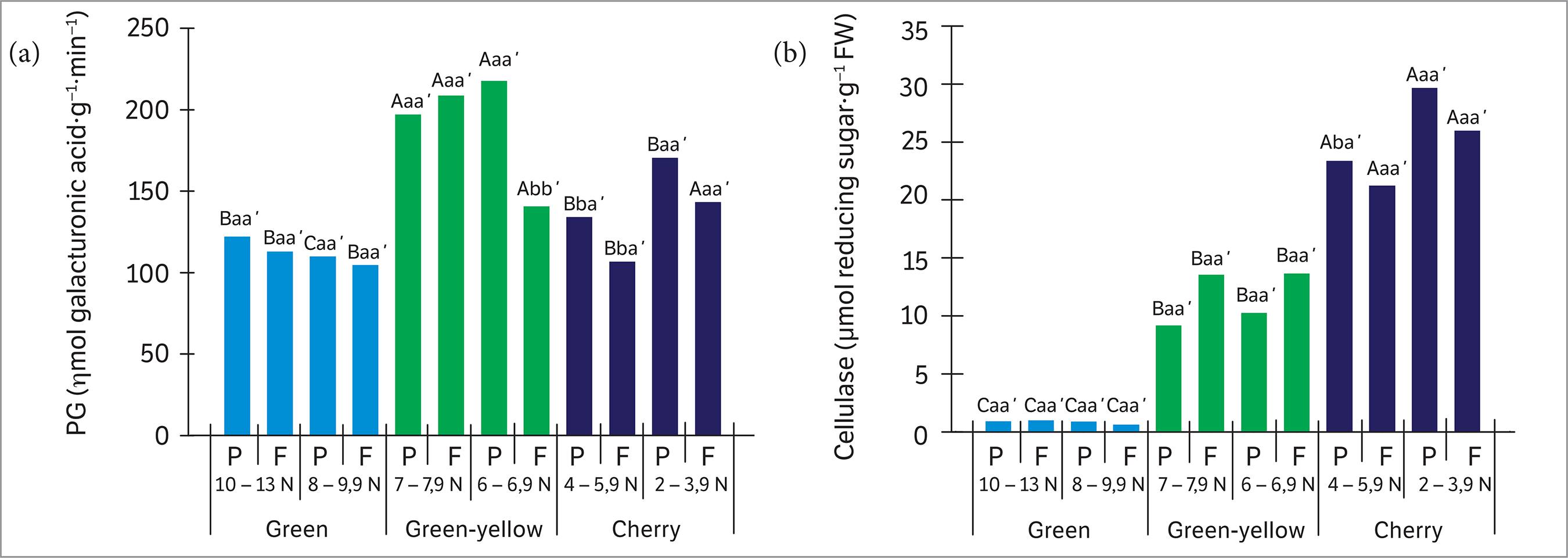
The highest levels of PG activity (Figure 5a) were found in the green-yellow stage, regardless of FDF and organ, and in the cherry stage, though only for minor FDF and only in the fruit. Cellulase activity (Figure 5b) also showed significant differences between the maturation stages, with the highest values observed in the cherry stage, regardless of FDF and organ. Statistical differences in detachment force were only observed in the cherry stage (both organs), with the highest cellulase activity associated with less force.
Activity of polygalacturonase (a) and cellulase (b) in the peduncles (P) — sample “c” — and fruit (F) — sample “d” — at different maturation stages and detachment forces. Capital letters compare the maturation stages in each detachment force (minor and major) and each organ; lowercase letters compare the detachment forces in each maturation stage and each organ; and lowercase letters followed by apostrophe compare the organs (P and F) in each detachment force and each maturation stage. Different letters indicate significant differences with 95% of probability.
PG is a pectinase associated with cell wall loosening, while cellulase promotes cell wall lysis in a process coordinated throughout fruit maturation (Bonghi et al. 1992Bonghi, C., Rascio, N., Ramina, A. and Casadoro, G. (1992). Cellulase and polygalacturonase involvement in the abscission of leaf and fruit explants of peach. Plant Molecular Biology, 20, 839-248. http://dx.doi.org/10.1007/BF00027155.
http://dx.doi.org/10.1007/BF00027155...
; Goulao and Oliveira 2008Goulao, L. F. and Oliveira, C. M. (2008). Cell wall modifications during fruit ripening: when a fruit is not the fruit. Trends in Food Science & Technology, 19, 4-25. http://dx.doi.org/10.1016/j.tifs.2007.07.002.
http://dx.doi.org/10.1016/j.tifs.2007.07...
). Disruption of the cell wall by enzymatic action begins with the digestion of pectic polysaccharides containing polygalacturonic acid. Firstly, pectinase (PG) leads to the hydrolysis ofα 1-4 bonds between galacturonic acid residues in the cell wall. Cellulase then cleaves the β 1-4 glycolytic bonds of cellulose or carboxymethyl cellulose molecules, which are present in the amorphous region of the cell wall (Rodionova et al. 2001Rodionova, N. A., Dubovaya, N. V., Martinovich, L. I. and Bezborodov, A. M. (2001). Formation of extracellular enzyme systems during Geotrichum candidum 3C growing on the cell walls isolated from cereal grains. Applied Biochemistry and Microbiology, 37, 562-565. http://dx.doi.org/10.1023/B:RUPP.0000003277.22914.6c.
http://dx.doi.org/10.1023/B:RUPP.0000003...
). Studies about fruit ripening reveal that, during maturation process, PG promotes cell wall loosening, allowing greater access of cellulase, which, in turn, more efficiently degrades the cell wall. Thus, there is one possible hypothesis for coffee fruit ripening, since we observed increased activity of both enzymes through the transition from green-yellow to cherry stage.
The increase in cellulase activity throughout fruit maturation stages has been reported where it is strongly associated with the abscission of the fruits (Wu et al. 2008Wu, Y., Deng, Y. and Li, Y. (2008). Changes in enzyme activities in abscission zone and berry drop of “Kyoho” grapes under high O2 or CO2 atmospheric storage. LWT - Food Science and Technology, 41, 175-179. http://dx.doi.org/10.1016/j.lwt.2007.01.015.
http://dx.doi.org/10.1016/j.lwt.2007.01....
). These enzymes drive the fruit abscission process through dissolution of the middle lamella and cell wall degradation.
The activation of the cell wall-degrading enzymes may be triggered by the presence of ROS. For example, H2O2 is known to act as a signaling molecule for activation of these enzymes (Sakamoto et al. 2008Sakamoto, M., Munemura, I., Tomita, R. and Kobayashi, K. (2008). Involvement of hydrogen peroxide in leaf abscission signaling, revealed by analysis with an in vitro abscission system in Capsicum plants. The Plant Journal, 58, 13-27. http://dx.doi.org/10.1111/j.1365-313X.2008.03577.x.
http://dx.doi.org/10.1111/j.1365-313X.20...
). In our study, H2O2 levels (Figure 6a) were higher in green and green-yellow stages, in peduncles and fruits collected by smaller force and in the cherry stage (regardless of the force and organ). Significant differences in H2O2 levels were observed between all maturation stages and sampling forces, with the highest overall values observed in fruits.
Hydrogen peroxide content (a) and lipid peroxidation levels (b) in the peduncles (P) — sample “c” — and fruit (F) — sample “d” — at different maturation stages and detachment forces. Capital letters compare the maturation stages in each detachment force (minor and major) and each organ; lowercase letters compare the detachment forces in each maturation stage and each organ; and lowercase letters followed by apostrophe compare the organs (P and F) in each detachment force and each maturation stage. Different letters indicate significant differences with 95% of probability.
Lipid peroxidation (Figure 6b) was higher in the later stages of maturation. The highest average was obtained from peduncles and fruits harvested with a smaller force, both at the green and the green-yellow stages. The highest means were found in the cherry stage, regardless of detachment force or organ. Within the cherry stage, higher levels of lipid peroxidation were recorded in fruits obtained with greater detachment force.
H2O2 is a key regulator of different processes, including fruit maturation and senescence of flowers and leaves (Mittler et al. 2004Mittler, R., Vanderauwera, S., Gollery, M. and van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends in Plant Sciences, 9, 490-498. http://dx.doi.org/10.1016/j.tplants.2004.08.009.
http://dx.doi.org/10.1016/j.tplants.2004...
; Bright et al. 2006Bright, J., Desikan, R., Hancock, J. T., Weir, I. S. and Neill, S. J. (2006). ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. The Plant Journal, 45, 113-122. http://dx.doi.org/10.1111/j.1365-313X.2005.02615.x.
http://dx.doi.org/10.1111/j.1365-313X.20...
). Moreover, the degradation of cellular components and disruption of membranes during fruit development are triggered by ROS accumulation. Thus, maturation and senescence are recognized as oxidative processes (Awad et al. 2011Awad, M. A., Al-Qurashi, A. D. and Mohamed, S. A. (2011). Antioxidant capacity, antioxidant compounds and antioxidant enzyme activities in five date cultivars during development and ripening. Scientia Horticulturae, 129, 688-693. http://dx.doi.org/10.1016/j.scienta.2011.05.019.
http://dx.doi.org/10.1016/j.scienta.2011...
). H2O2, together with ethylene, regulates cellulase expression (Sakamoto et al. 2008Sakamoto, M., Munemura, I., Tomita, R. and Kobayashi, K. (2008). Involvement of hydrogen peroxide in leaf abscission signaling, revealed by analysis with an in vitro abscission system in Capsicum plants. The Plant Journal, 58, 13-27. http://dx.doi.org/10.1111/j.1365-313X.2008.03577.x.
http://dx.doi.org/10.1111/j.1365-313X.20...
). For example, in Capsicum chinense and C. baccato, the final abscission of leaves was associated with increased H2O2 production, which degrades cell walls by cleaving polysaccharides (Sakamoto et al. 2008Sakamoto, M., Munemura, I., Tomita, R. and Kobayashi, K. (2008). Involvement of hydrogen peroxide in leaf abscission signaling, revealed by analysis with an in vitro abscission system in Capsicum plants. The Plant Journal, 58, 13-27. http://dx.doi.org/10.1111/j.1365-313X.2008.03577.x.
http://dx.doi.org/10.1111/j.1365-313X.20...
).
The intensity of oxidative stress can be measured by the extent of lipid peroxidation: imbalances between the production and removal of ROS can result in damage to the membranes due to ROS (Sreelatha and Padma 2009Sreelatha, S. and Padma, P. R. (2009). Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods for Human Nutrition, 64, 303-311. http://dx.doi.org/10.1007/s11130-009-0141-0.
http://dx.doi.org/10.1007/s11130-009-014...
). In our study, higher H2O2 concentrations were found in samples using greater detachment force in the cherry stage. This molecule is probably a signal for the activation of cell wall-degrading enzymes, resulting in cell wall loosening and, consequently, a smaller fruit-detachment force.
During coffee ripening, the damage caused by the ROS (H2O2) resulted in increased lipid peroxidation in the cherry-stage fruits. Finally, our data suggest that maturation of coffee fruits of the cv. “Icatu amarelo” is characterized by morphological and enzymatic changes associated to an increase in oxidative stress.
CONCLUSION
Coffea arabica cv. “Icatu amarelo” does not have a defined abscission zone between peduncle and fruit. Nevertheless, cell wall loosening occurs during maturation in random cells due to the activity of cell wall-degrading enzymes and the action of ROS. The result of these processes is that a smaller detachment force is required in cherry-stage fruits.
REFERENCES
- Awad, M. A., Al-Qurashi, A. D. and Mohamed, S. A. (2011). Antioxidant capacity, antioxidant compounds and antioxidant enzyme activities in five date cultivars during development and ripening. Scientia Horticulturae, 129, 688-693. http://dx.doi.org/10.1016/j.scienta.2011.05.019
» http://dx.doi.org/10.1016/j.scienta.2011.05.019 - Bonghi, C., Rascio, N., Ramina, A. and Casadoro, G. (1992). Cellulase and polygalacturonase involvement in the abscission of leaf and fruit explants of peach. Plant Molecular Biology, 20, 839-248. http://dx.doi.org/10.1007/BF00027155
» http://dx.doi.org/10.1007/BF00027155 - Bright, J., Desikan, R., Hancock, J. T., Weir, I. S. and Neill, S. J. (2006). ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. The Plant Journal, 45, 113-122. http://dx.doi.org/10.1111/j.1365-313X.2005.02615.x
» http://dx.doi.org/10.1111/j.1365-313X.2005.02615.x - Buege, J. A. and Aust, S. D. (1978). Microsomal lipid peroxidation. Methods in Enzymology, 52, 302-310. http://dx.doi.org/10.1016/S0076-6879(78)52032-6
» http://dx.doi.org/10.1016/S0076-6879(78)52032-6 - Buescher, R. W. and Furmanski, R. J. (1978). Role of pectinesterase and polygalacturonase in the formation of woolliness in peaches. Journal of Food Science, 43, 264-266. http://dx.doi.org/10.1111/j.1365-2621.1978.tb09788.x
» http://dx.doi.org/10.1111/j.1365-2621.1978.tb09788.x - Chaves Filho, J. T. (2007). Novos paradigmas na fisiologia do cafeeiro. Documentos- IAC, 80, 67-74.
- Estornell, L. H., Agustí, J., Merelo, P., Talón, M. and Tadeo, F. R. (2013). Elucidating mechanisms underlying organ abscission. Plant Science, 199-200, 48-60. http://dx.doi.org/10.1016/j.plantsci.2012.10.008
» http://dx.doi.org/10.1016/j.plantsci.2012.10.008 - Ferreira, D. F. (2011). Sisvar: a computer statistical analysis system. Ciência e Agrotecnologia, 35, 1039-1042. http://dx.doi.org/10.1590/S1413-70542011000600001
» http://dx.doi.org/10.1590/S1413-70542011000600001 - Goulao, L. F. and Oliveira, C. M. (2008). Cell wall modifications during fruit ripening: when a fruit is not the fruit. Trends in Food Science & Technology, 19, 4-25. http://dx.doi.org/10.1016/j.tifs.2007.07.002
» http://dx.doi.org/10.1016/j.tifs.2007.07.002 - Lewis, M. W., Leslie, M. E. and Liljegren, S. J. (2006). Plant separation: 50 ways to leave your mother. Current Opinion in Plant Biology, 9, 59-65. http://dx.doi.org/10.1016/j.pbi.2005.11.009
» http://dx.doi.org/10.1016/j.pbi.2005.11.009 - Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426-428. http://dx.doi.org/10.1021/ac60147a030
» http://dx.doi.org/10.1021/ac60147a030 - Mittler, R., Vanderauwera, S., Gollery, M. and van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends in Plant Sciences, 9, 490-498. http://dx.doi.org/10.1016/j.tplants.2004.08.009
» http://dx.doi.org/10.1016/j.tplants.2004.08.009 - Mondal, K., Malhotra, S. P., Jain, V. and Singh, R. (2009). Oxidative stress and antioxidant systems in Guava (Psidium guajava L.) fruits during ripening. Physiology and Molecular Biology of Plants, 15, 327-334. http://dx.doi.org/10.1007/s12298-009-0037-3
» http://dx.doi.org/10.1007/s12298-009-0037-3 - Nelson, N. A. (1944). A photometric adaptation of Somogyi method for the determination of glucose. The Journal of Biological Chemistry, 153, 375-380.
- O’Brien, T. P., Feder, N. and McCully, M. E. (1964). Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma, 59, 368-373. http://dx.doi.org/10.1007/BF01248568
» http://dx.doi.org/10.1007/BF01248568 - Parra-Lobato, M. C. and Gomez-Jimenez, M. C. (2011). Polyamine-induced modulation of genes involved in ethylene biosynthesis and signalling pathways and nitric oxide production during olive mature fruit abscission. Journal of Experimental Botany, 62, 4447-4465. http://dx.doi.org/10.1093/jxb/err124
» http://dx.doi.org/10.1093/jxb/err124 - Rodionova, N. A., Dubovaya, N. V., Martinovich, L. I. and Bezborodov, A. M. (2001). Formation of extracellular enzyme systems during Geotrichum candidum 3C growing on the cell walls isolated from cereal grains. Applied Biochemistry and Microbiology, 37, 562-565. http://dx.doi.org/10.1023/B:RUPP.0000003277.22914.6c
» http://dx.doi.org/10.1023/B:RUPP.0000003277.22914.6c - Sakamoto, M., Munemura, I., Tomita, R. and Kobayashi, K. (2008). Involvement of hydrogen peroxide in leaf abscission signaling, revealed by analysis with an in vitro abscission system in Capsicum plants. The Plant Journal, 58, 13-27. http://dx.doi.org/10.1111/j.1365-313X.2008.03577.x
» http://dx.doi.org/10.1111/j.1365-313X.2008.03577.x - Scott, A. J., Knott, M. (1974). A cluster analysis method for grouping means in the analysis of variance. Biometrics, 30, 507-512. http://dx.doi.org/10.2307/2529204
» http://dx.doi.org/10.2307/2529204 - Silva, F. C., Silva, F. M., Alves, M. C., Barros, M. M. and Sales, R. S. (2010). Comportamento da força de desprendimento dos frutos de cafeeiros ao longo do período de colheita. Ciência e Agrotecnologia, 34, 468-474. http://dx.doi.org/10.1590/S1413-70542010000200028
» http://dx.doi.org/10.1590/S1413-70542010000200028 - Silva, F. C., Silva, F. M., Silva, A. C., Barros, M. M. and Palma, M. A. Z. (2013). Desempenho operacional da colheita mecanizada e seletiva do café em função da força de desprendimento dos frutos. Coffee Science, 8, 53-60.
- Silva, F. M., Silva, F. O. E. and Miranda, N. B. (2014). Mecanização da colheita viabiliza cafeicultor. Granja, 784, 60-62.
- Sreelatha, S. and Padma, P. R. (2009). Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods for Human Nutrition, 64, 303-311. http://dx.doi.org/10.1007/s11130-009-0141-0
» http://dx.doi.org/10.1007/s11130-009-0141-0 - Velikova, V., Yordanov, I. and Edreva, A. (2000). Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Science, 151, 59-66. http://dx.doi.org/10.1016/S0168-9452(99)00197-1
» http://dx.doi.org/10.1016/S0168-9452(99)00197-1 - Vilas-Boas, E. V. B., Chitarra, A. B. and Chitarra, M. I. F. (1996). Modificações pós-colheita de bananas ‘Prata’ y-irradiada. Pesquisa Agropecuária Brasileira, 31, 599-607.
- Walker, D., Ledesma, P., Delgado, O. D. and Breccia, J. D. (2006). High endo β-1,4-D-glucanase activity in a broad pH range from the alkali-tolerant Nocardipsis sp. SES28. World Journal of Microbiology and Biotechnology, 22, 761-764. http://dx.doi.org/10.1007/s11274-005-9097-x
» http://dx.doi.org/10.1007/s11274-005-9097-x - Wu, Y., Deng, Y. and Li, Y. (2008). Changes in enzyme activities in abscission zone and berry drop of “Kyoho” grapes under high O2 or CO2 atmospheric storage. LWT - Food Science and Technology, 41, 175-179. http://dx.doi.org/10.1016/j.lwt.2007.01.015
» http://dx.doi.org/10.1016/j.lwt.2007.01.015
Publication Dates
-
Publication in this collection
06 Oct 2016 -
Date of issue
Oct-Dec 2016
History
-
Received
26 Nov 2015 -
Accepted
06 Mar 2016