Abstract
Antibiotic resistance is one of the greatest challenges to treat bacterial infections worldwide, leading to increase in medical expenses, prolonged hospital stay and increased mortality. The use of blue light has been suggested as an innovative alternative to overcome this problem. In this study we analyzed the antibacterial effect of blue light using low emission parameters on Staphylococcus aureus cultures. In vitro bacterial cultures were used in two experimental approaches. The first approach included single or fractionated blue light application provided by LED emitters (470 nm), with the following fluencies: 16.29, 27.16 and 54.32 J/cm2. For the second approach a power LED (470 nm) was used to deliver 54.32 J/cm2 fractionated in 3 applications. Our results demonstrated that bacterial cultures exposed to fractionated blue light radiation exhibited significantly smaller sizes colonies than the control group after 24 h incubation, however the affected bacteria were able to adapt and continue to proliferate after prolonged incubation time. We could conclude that the hypothetical clinical use of low fluencies of blue light as an antibacterial treatment is risky, since its action is not definitive and proves to be ineffective at least for the strain used in this study.
Keywords:
blue light; photobiomodulation; Staphylococcus aureus; in vitro study
Resumo
A resistência a antibióticos é um dos maiores desafios para o tratamento de infecções bacterianas em todo o mundo, levando ao aumento de despesas médicas, prolongamento da internação hospitalar e aumento da mortalidade. O uso da luz azul tem sido sugerido como uma alternativa inovadora para superar esse problema. Neste estudo, analisamos o efeito antibacteriano da luz azul usando parâmetros de baixa emissão em culturas de Staphylococcus aureus. Culturas bacterianas foram usadas em duas abordagens experimentais in vitro. A primeira abordagem incluiu o uso da aplicação única ou fracionada de luz azul fornecida por emissores de LED (470 nm), com as seguintes fluências: 16,29, 27,16 e 54,32 J/cm2. Para a segunda abordagem, um LED de potência (470 nm) foi usado para fornecer 54,32 J/cm2 fracionado em 3 aplicações. Nossos resultados demonstraram que as culturas bacterianas expostas à radiação de luz azul fracionada exibiram colônias de tamanhos significativamente menores do que o grupo controle após 24 h de incubação, no entanto, as bactérias afetadas foram capazes de se adaptar e continuar a proliferar após um tempo prolongado de incubação. Podemos concluir que o uso clínico hipotético de baixas fluências de luz azul como tratamento antibacteriano é arriscado, pois sua ação não é definitiva e mostra-se ineficaz, pelo menos para a cepa utilizada neste estudo.
Palavras-chave:
luz azul; fotobiomodulação; Staphylococcus aureus; estudo in vitro
1. Introduction
It has been demonstrated that several bacterial species are able to develop resistance to pharmacological treatment due to inappropriate and longstanding use of antibiotic drugs (Dai et al., 2012DAI, T., GUPTA, A., MURRAY, C.K., VRAHAS, M.S., TEGOS, G.P. and HAMBLIN, M.R., 2012. Blue light for infectious diseases: propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resistance Updates, vol. 15, no. 4, pp. 223-236. http://dx.doi.org/10.1016/j.drup.2012.07.001. PMid:22846406.
http://dx.doi.org/10.1016/j.drup.2012.07...
; Bumah et al., 2013BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S.E. and ENWEMEKA, C.S., 2013. Wavelength and bacterial density influence the bactericidal effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA). Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 547-553. http://dx.doi.org/10.1089/pho.2012.3461. PMid:23621894.
http://dx.doi.org/10.1089/pho.2012.3461...
; Meurer et al., 2019MEURER, L., PAYNE, W. and GUFFEY, J.S., 2019. Visible light as an inhibitor of Camplyobacter jejuni. International Journal of Antimicrobial Agents, vol. 55, no. 1, pp. 105818. PMid:31593764.; Rupel et al., 2019RUPEL, K., ZUPIN, L., OTTAVIANI, G., BERTANI, I., MARTINELLI, V., PORRELLI, D., VODRET, S., VUERICH, R., PASSOS DA SILVA, D., BUSSANI, R., CROVELLA, S., PARSEK, M., VENTURI, V., DI LENARDA, R., BIASOTTO, M. and ZACCHIGNA, S., 2019. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. Biofilms and Microbiomes, vol. 5, no. 29, pp. 1-11. http://dx.doi.org/10.1038/s41522-019-0102-9. PMid:31602310.
http://dx.doi.org/10.1038/s41522-019-010...
). Staphylococcus aureus (S. aureus) is a Gram-positive aerobic bacterium, which presents a highly evolved capacity of developing resistance to antimicrobial drugs. Additionally, it is an important etiological agent in skin wound contaminations, often evolving to generalized infections (Enwemeka et al., 2009ENWEMEKA, C.S., WILLIAMS, D., ENWEMEKA, S.K., HOLLOSI, S. and YENS, D., 2009. Blue 470-nm light kills Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomedicine and Laser Surgery, vol. 27, no. 2, pp. 221-226. http://dx.doi.org/10.1089/pho.2008.2413. PMid:19196103.
http://dx.doi.org/10.1089/pho.2008.2413...
; Bumah et al., 2013BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S.E. and ENWEMEKA, C.S., 2013. Wavelength and bacterial density influence the bactericidal effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA). Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 547-553. http://dx.doi.org/10.1089/pho.2012.3461. PMid:23621894.
http://dx.doi.org/10.1089/pho.2012.3461...
, 2015BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S. and ENWEMEKA, C.S., 2015. Optimization of the antimicrobial effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers in Surgery and Medicine, vol. 47, no. 3, pp. 266-272. http://dx.doi.org/10.1002/lsm.22327. PMid:25639752.
http://dx.doi.org/10.1002/lsm.22327...
; Sousa et al., 2015SOUSA, N.T.A., SANTOS, M.F., GOMES, R.C., BRANDINO, H.E., MARTINEZ, R. and GUIRRO, R.R.J., 2015. Blue laser inhibits bacterial growth of Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. Photomedicine and Laser Surgery, vol. 33, no. 5, pp. 278-282. http://dx.doi.org/10.1089/pho.2014.3854. PMid:25954830.
http://dx.doi.org/10.1089/pho.2014.3854...
; Freitas et al., 2018FREITAS, C.H., MENDES, J.F., VILLARREAL, P.V., SANTOS, P.R., GONÇALVES, C.L., GONZALES, H.L. and NASCENTE, P.S., 2018. Identification and antimicrobial suceptibility profile of bacteria causing bovine mastitis from dairy farms in Pelotas, Rio Grande do Sul. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 78, no. 4, pp. 661-666. http://dx.doi.org/10.1590/1519-6984.170727. PMid:29319754.
http://dx.doi.org/10.1590/1519-6984.1707...
; Ferrer-Espada et al., 2019FERRER-ESPADA, R., WANG, Y., GOH, X.S. and DAI, T., 2019. Antimicrobial blue light inactivation of microbial isolates in biofilms. Lasers in Surgery and Medicine, In press. http://dx.doi.org/10.1002/lsm.23159. PMid:31536154.
http://dx.doi.org/10.1002/lsm.23159...
; Rupel et al., 2019RUPEL, K., ZUPIN, L., OTTAVIANI, G., BERTANI, I., MARTINELLI, V., PORRELLI, D., VODRET, S., VUERICH, R., PASSOS DA SILVA, D., BUSSANI, R., CROVELLA, S., PARSEK, M., VENTURI, V., DI LENARDA, R., BIASOTTO, M. and ZACCHIGNA, S., 2019. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. Biofilms and Microbiomes, vol. 5, no. 29, pp. 1-11. http://dx.doi.org/10.1038/s41522-019-0102-9. PMid:31602310.
http://dx.doi.org/10.1038/s41522-019-010...
).
Bacterial resistance and reproduction speed make it difficult to deal with infections caused by bacterial microorganisms and this fact leads to increased mortality risk to the patient as well as increased treatment cost and prolonged hospital stay. Therefore, there is an urgent need for safe, efficient and cheap therapeutic alternatives to combat bacterial agents like S. aureus (Enwemeka et al., 2009ENWEMEKA, C.S., WILLIAMS, D., ENWEMEKA, S.K., HOLLOSI, S. and YENS, D., 2009. Blue 470-nm light kills Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomedicine and Laser Surgery, vol. 27, no. 2, pp. 221-226. http://dx.doi.org/10.1089/pho.2008.2413. PMid:19196103.
http://dx.doi.org/10.1089/pho.2008.2413...
; Bumah et al., 2015BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S. and ENWEMEKA, C.S., 2015. Optimization of the antimicrobial effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers in Surgery and Medicine, vol. 47, no. 3, pp. 266-272. http://dx.doi.org/10.1002/lsm.22327. PMid:25639752.
http://dx.doi.org/10.1002/lsm.22327...
; Rupel et al., 2019RUPEL, K., ZUPIN, L., OTTAVIANI, G., BERTANI, I., MARTINELLI, V., PORRELLI, D., VODRET, S., VUERICH, R., PASSOS DA SILVA, D., BUSSANI, R., CROVELLA, S., PARSEK, M., VENTURI, V., DI LENARDA, R., BIASOTTO, M. and ZACCHIGNA, S., 2019. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. Biofilms and Microbiomes, vol. 5, no. 29, pp. 1-11. http://dx.doi.org/10.1038/s41522-019-0102-9. PMid:31602310.
http://dx.doi.org/10.1038/s41522-019-010...
).
Photobiomodulation using wavelength between violet and blue spectrum (405-470 nm) has been receiving great attention in the last decade and is considered an example of antibacterial therapy. Thus, the effect of blue light on different species of bacteria has been the subject of several studies (Feuerstein, 2012FEUERSTEIN, O., 2012. Light therapy: complementary antibacterial treatment of oral biofilm. Advances in Dental Research, vol. 24, no. 2, pp. 103-107. http://dx.doi.org/10.1177/0022034512449469. PMid:22899690.
http://dx.doi.org/10.1177/00220345124494...
; Bumah et al., 2013BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S.E. and ENWEMEKA, C.S., 2013. Wavelength and bacterial density influence the bactericidal effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA). Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 547-553. http://dx.doi.org/10.1089/pho.2012.3461. PMid:23621894.
http://dx.doi.org/10.1089/pho.2012.3461...
; Dai et al., 2013DAI, T., GUPTA, A., HUANG, Y.Y., SHERWOOD, M.E., MURRAY, C.K., VRAHAS, M.S., KIELIAN, T. and HAMBLIN, M.R., 2013. Blue light eliminates community-acquired Methicillin-resistant Staphylococcus aureus in infected mouse skin abrasions. Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 531-538. http://dx.doi.org/10.1089/pho.2012.3365. PMid:23406384.
http://dx.doi.org/10.1089/pho.2012.3365...
; Guffey et al., 2013GUFFEY, J.S., PAYNE, W., JONES, T. and MARTIN, K., 2013. Evidence of resistance development by Staphylococcus aureus to an in vitro, multiple stage application of 405 nm light from a supraluminous diode array. Photomedicine and Laser Surgery, vol. 31, no. 4, pp. 179-182. http://dx.doi.org/10.1089/pho.2012.3450. PMid:23484587.
http://dx.doi.org/10.1089/pho.2012.3450...
; Sousa et al., 2015SOUSA, N.T.A., SANTOS, M.F., GOMES, R.C., BRANDINO, H.E., MARTINEZ, R. and GUIRRO, R.R.J., 2015. Blue laser inhibits bacterial growth of Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. Photomedicine and Laser Surgery, vol. 33, no. 5, pp. 278-282. http://dx.doi.org/10.1089/pho.2014.3854. PMid:25954830.
http://dx.doi.org/10.1089/pho.2014.3854...
; Wang et al., 2017WANG, Y., WANG, Y., WANG, Y., MURRAY, C.K., HAMBLIN, M.R., HOOPER, D.C. and DAI, T., 2017. Antimicrobial blue light inactivation of pathogenic microbes: state of the art. Drug Resistance Updates, vol. 33, no. 35, pp. 1-56. http://dx.doi.org/10.1016/j.drup.2017.10.002. PMid:29145971.
http://dx.doi.org/10.1016/j.drup.2017.10...
, Ferrer-Espada et al., 2019FERRER-ESPADA, R., WANG, Y., GOH, X.S. and DAI, T., 2019. Antimicrobial blue light inactivation of microbial isolates in biofilms. Lasers in Surgery and Medicine, In press. http://dx.doi.org/10.1002/lsm.23159. PMid:31536154.
http://dx.doi.org/10.1002/lsm.23159...
; Meurer et al., 2019MEURER, L., PAYNE, W. and GUFFEY, J.S., 2019. Visible light as an inhibitor of Camplyobacter jejuni. International Journal of Antimicrobial Agents, vol. 55, no. 1, pp. 105818. PMid:31593764.; Rupel et al., 2019RUPEL, K., ZUPIN, L., OTTAVIANI, G., BERTANI, I., MARTINELLI, V., PORRELLI, D., VODRET, S., VUERICH, R., PASSOS DA SILVA, D., BUSSANI, R., CROVELLA, S., PARSEK, M., VENTURI, V., DI LENARDA, R., BIASOTTO, M. and ZACCHIGNA, S., 2019. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. Biofilms and Microbiomes, vol. 5, no. 29, pp. 1-11. http://dx.doi.org/10.1038/s41522-019-0102-9. PMid:31602310.
http://dx.doi.org/10.1038/s41522-019-010...
; Schmid et al., 2019SCHMID, J., HOENES, K., VATTER, P. and HESSLING, M., 2019. Antimicrobial effect of visible light-photoinactivation of Legionella rubrilucens by irradiation at 450, 470, and 620 nm. Antibiotics (Basel, Switzerland), vol. 8, no. 187, pp. pii:E187. http://dx.doi.org/10.3390/antibiotics8040187. PMid:31618994.
http://dx.doi.org/10.3390/antibiotics804...
). The blue light antimicrobial effect has been observed using different experiment protocols, however, the true mechanism of action is still not fully understood (Chui et al., 2012CHUI, C., HIRATSUKA, K., AOKI, A., TAKEUCHI, Y., ABIKO, Y. and IZUMI, Y., 2012. Blue LED inhibits the growth of Porphyromonas gingivalis by suppressing the expression of genes associated with DNA replication and cell division. Lasers in Surgery and Medicine, vol. 44, no. 10, pp. 856-864. http://dx.doi.org/10.1002/lsm.22090. PMid:23129157.
http://dx.doi.org/10.1002/lsm.22090...
; Dai et al., 2013DAI, T., GUPTA, A., HUANG, Y.Y., SHERWOOD, M.E., MURRAY, C.K., VRAHAS, M.S., KIELIAN, T. and HAMBLIN, M.R., 2013. Blue light eliminates community-acquired Methicillin-resistant Staphylococcus aureus in infected mouse skin abrasions. Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 531-538. http://dx.doi.org/10.1089/pho.2012.3365. PMid:23406384.
http://dx.doi.org/10.1089/pho.2012.3365...
; Bumah et al., 2015BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S. and ENWEMEKA, C.S., 2015. Optimization of the antimicrobial effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers in Surgery and Medicine, vol. 47, no. 3, pp. 266-272. http://dx.doi.org/10.1002/lsm.22327. PMid:25639752.
http://dx.doi.org/10.1002/lsm.22327...
; Wang et al., 2017WANG, Y., WANG, Y., WANG, Y., MURRAY, C.K., HAMBLIN, M.R., HOOPER, D.C. and DAI, T., 2017. Antimicrobial blue light inactivation of pathogenic microbes: state of the art. Drug Resistance Updates, vol. 33, no. 35, pp. 1-56. http://dx.doi.org/10.1016/j.drup.2017.10.002. PMid:29145971.
http://dx.doi.org/10.1016/j.drup.2017.10...
; Rupel et al., 2019RUPEL, K., ZUPIN, L., OTTAVIANI, G., BERTANI, I., MARTINELLI, V., PORRELLI, D., VODRET, S., VUERICH, R., PASSOS DA SILVA, D., BUSSANI, R., CROVELLA, S., PARSEK, M., VENTURI, V., DI LENARDA, R., BIASOTTO, M. and ZACCHIGNA, S., 2019. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. Biofilms and Microbiomes, vol. 5, no. 29, pp. 1-11. http://dx.doi.org/10.1038/s41522-019-0102-9. PMid:31602310.
http://dx.doi.org/10.1038/s41522-019-010...
; Schmid et al., 2019SCHMID, J., HOENES, K., VATTER, P. and HESSLING, M., 2019. Antimicrobial effect of visible light-photoinactivation of Legionella rubrilucens by irradiation at 450, 470, and 620 nm. Antibiotics (Basel, Switzerland), vol. 8, no. 187, pp. pii:E187. http://dx.doi.org/10.3390/antibiotics8040187. PMid:31618994.
http://dx.doi.org/10.3390/antibiotics804...
). Furthermore, the optimal emission parameter values to be used, especially regarding irradiance and fluence, are currently in discussion among several researchers. However, there is controversy over the use of low blue light emission parameters as a topical antimicrobial therapy (Chui et al., 2012CHUI, C., HIRATSUKA, K., AOKI, A., TAKEUCHI, Y., ABIKO, Y. and IZUMI, Y., 2012. Blue LED inhibits the growth of Porphyromonas gingivalis by suppressing the expression of genes associated with DNA replication and cell division. Lasers in Surgery and Medicine, vol. 44, no. 10, pp. 856-864. http://dx.doi.org/10.1002/lsm.22090. PMid:23129157.
http://dx.doi.org/10.1002/lsm.22090...
; Bumah et al., 2015BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S. and ENWEMEKA, C.S., 2015. Optimization of the antimicrobial effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers in Surgery and Medicine, vol. 47, no. 3, pp. 266-272. http://dx.doi.org/10.1002/lsm.22327. PMid:25639752.
http://dx.doi.org/10.1002/lsm.22327...
; Sousa et al., 2015SOUSA, N.T.A., SANTOS, M.F., GOMES, R.C., BRANDINO, H.E., MARTINEZ, R. and GUIRRO, R.R.J., 2015. Blue laser inhibits bacterial growth of Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. Photomedicine and Laser Surgery, vol. 33, no. 5, pp. 278-282. http://dx.doi.org/10.1089/pho.2014.3854. PMid:25954830.
http://dx.doi.org/10.1089/pho.2014.3854...
; Meurer et al., 2019MEURER, L., PAYNE, W. and GUFFEY, J.S., 2019. Visible light as an inhibitor of Camplyobacter jejuni. International Journal of Antimicrobial Agents, vol. 55, no. 1, pp. 105818. PMid:31593764.; Schmid et al., 2019SCHMID, J., HOENES, K., VATTER, P. and HESSLING, M., 2019. Antimicrobial effect of visible light-photoinactivation of Legionella rubrilucens by irradiation at 450, 470, and 620 nm. Antibiotics (Basel, Switzerland), vol. 8, no. 187, pp. pii:E187. http://dx.doi.org/10.3390/antibiotics8040187. PMid:31618994.
http://dx.doi.org/10.3390/antibiotics804...
).
Thus, considering all the conflicting data reported in the literature the aim of this study was to verify the in vitro effect of blue light (470 nm) in S. aureus bacterial cultures by using low irradiance and fluence values at single and fractionated applications.
2. Material and Methods
2.1. Bacterial strain and cultures
S. aureus strain (ATCC 25923) was obtained from the American Type Culture Collection cultures. For the Experiment 1, the bacterium was inoculated in sterile saline solution, in order to obtain a scale of 0.5 McFarland turbidity standard (1.5 x 108 CFU/mL) and the streak was made using a sterile swab all over the surface of a 150 mm diameter sterile Petri dishes containing Mueller-Hinton agar. For the Experiment 2, serial dilution (1:10000) was made from the same scale as mentioned before, 100 μL of the diluted solution was pipetted into 55 mm diameter Petri dishes containing Mueller-Hinton agar and the streak was made using a Drigalski spatula.
2.2. Light emitters
A blue Light-Emitting Diode (LED), with a viewing angle of 30°, emitting a continuous beam of 470 nm wavelength, 0.247 cm2 beam area, 4.92 mW radiometric power, and irradiance of 19.92 mW/cm2 was used to irradiate each exposure area in Experiment 1. The LED was positioned at a distance of 2.13 cm, perpendicularly, from the bacterial culture surface. The exposure times adopted were 13.63, 22.72 and 45.45 min, respectively generating the following low fluencies: 16.29, 27.16 and 54.32 J/cm2.
In Experiment 2, a power LED (470 nm, continuous beam, 120° viewing angle, 850 mW radiometric power, 35.22 mW/cm2 irradiance) was used. It was positioned at a distance of 4.8 cm, perpendicularly, from the bacterial culture surface, which generated a beam area of 24.13 cm2 and allowed to cover the entire surface of the dish. The fluence used was 54.32 J/cm2, which was fractionated in 3 applications of 18.106 J/cm2. The exposure time of each application was 8.56 min (25.7 min total).
In both experiments, the temperature of the surface irradiated by the light was monitored, always remaining below 30º C, insufficient to generate any changes of the culture medium.
2.3. Experimental protocols
Experiment 1 was divided in Protocol 1 and Protocol 2. For both protocols, each exposure area was labelled with a dot (3 dots per dish). Each LED was positioned over a specific marked dot so that adequate fluence was applied to each one.
The Protocol 1 consisted of applying each fluence (16.29, 27.26 and 54.32 J/cm2) in each exposure area at a single time. Eight dishes were used: Experimental Group 1 (n = 4) and Control Group 1 (n = 4). Both experimental and control dishes were incubated for 24 hours at 36.5º C after the light exposure.
The Protocol 2 consisted of fractionating each fluence in three exposures, obtaining the following fractionated fluencies: 5.43, 9.053 and 18.106 J/cm2, each one was used at a specific area. Eight dishes were used: Experimental Group 2 (n = 4) and Control Group 2 (n = 4). Between each of the three exposures, it was allowed a 10-minute pause. Both experimental and control dishes were incubated for 24 hours at 36.5º C after the final light exposure.
In Experiment 2, twelve dishes were used: Experimental Group 3 (n = 6) and Control Group 3 (n = 6). A 1:10000 dilution from the 0.5 McFarland scale was obtained and seeded onto the dishes. For the experimental group, there was a fractional application of the fluence of 54.32 J/cm2 of the blue light (three exposures with a 10-minutes pause between each one).
Control dishes were always positioned side by side with experimental dishes from the same protocol, so that the environment influence was the same on them, but not being exposed to the light from the other dish.
At the end, digital photos of all dishes were taken after 24 and 48 hours of incubation. For Experiment 1, a comparison between experimental and control groups was established and the main goal was to verify the existence of inhibition halos on areas exposed to blue light. For Experiment 2, the photos were used for counting colonies and for measuring colony diameters using ImageJ software.
2.4. Statistical analysis
The statistical analyses were done using SPSS 17.0 software. The normality of the sample distribution was confirmed by the Shapiro-Wilk test. Data were expressed as mean ± standard deviation and Student's t-test for independent samples (p < 0.05) was used to compare experimental and control groups.
3. Results
Considering the seeding pattern and the incubated temperature, the bacterial growth was as expected in all of the control dishes of Experiment 1 (see Figure 1A). There were no inhibition areas on Experimental Group 1 dishes, revealing that single applications of low blue light fluencies do not induce growth inhibition in S. aureus cultures.
Agar plates representative of Experiment 1 (experimental group 2) showing S. aureus bacterial growth 24 hours after exposure to fractionated 16.29, 27.16 and 54.32 J/cm2 fluences of blue light. (A) Control Group 2 not exposed to blue light; (B) Inhibition halos, indicated by arrows, on Experimental Group 2. Inhibition from fractionated 54.32 J/cm2 fluence was the most effective.
On the other hand, on Experimental Group 2 dishes, there were discreet inhibition areas at the application dots previously labelled (see Figure 1B). The verified inhibition pattern suggests a fluence-dependent of the blue light antibacterial action, with 54.32 J/cm2 the most effective fluence.
In Experiment 2, a dilution was performed in order to obtain isolated colonies, as seen in Figure 2. The mean number of colonies for Experimental Group 3 was 127.16 ± 24.50, and the mean for Control Group 3 was 132.33 ± 13.80. There was no statistically significant difference between these counts (p = 0.706), indicating that the fractionated fluence of 54.32 J/cm2 was not enough to suppress the proliferation of S. aureus from lower density inoculum.
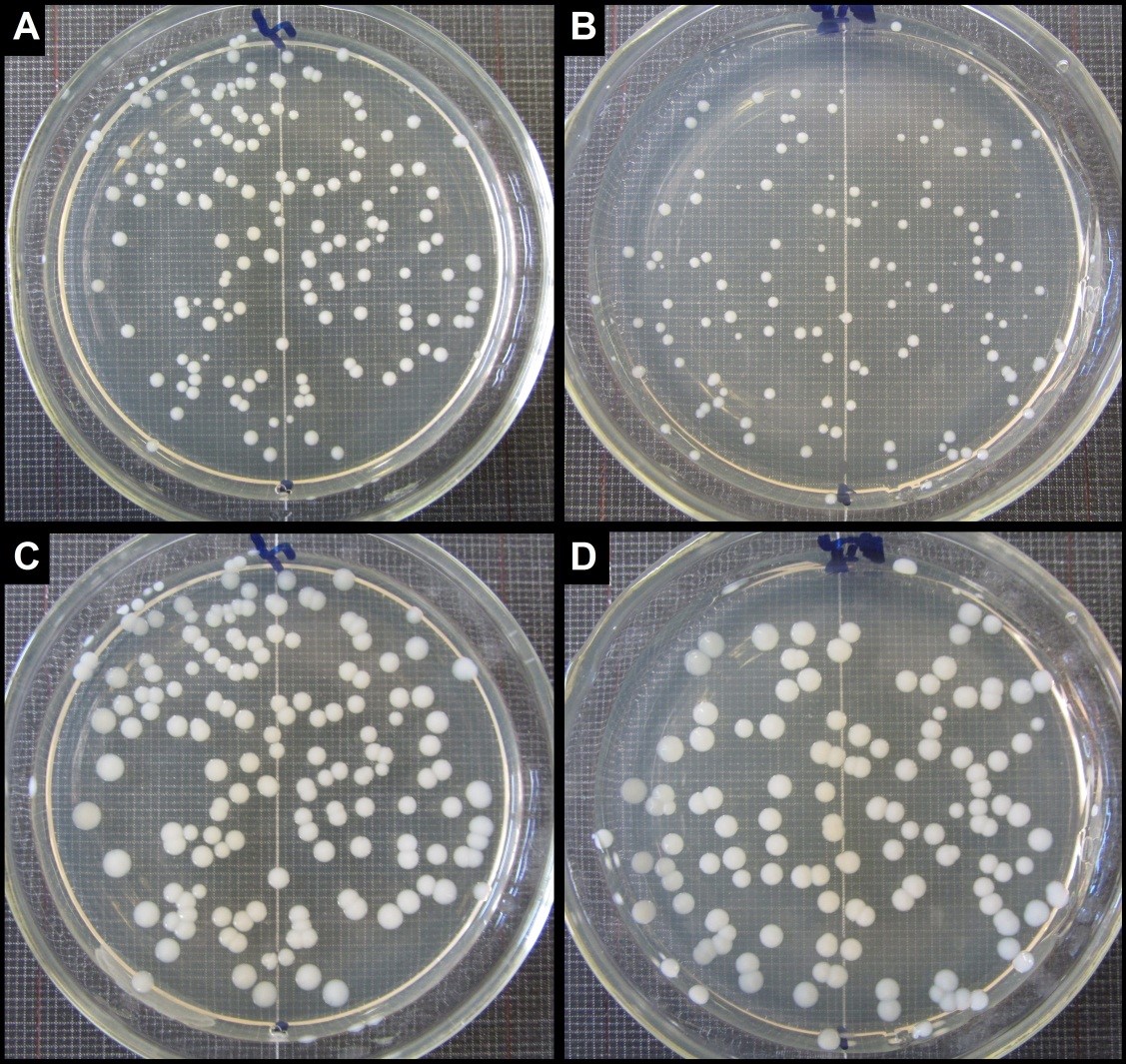
General appearance of the bacterial colonies grown on agar plates dishes of Experiment 2 after incubation for 24 and 48 hours. Cells were exposed to fractionated fluence of 54.32 J/cm2 (3 X 18.106 J/cm2). For both experimental and control group growth pattern occurred in a similar fashion, however cells exposed to blue light showed colonies with significantly smaller diameter. (A) Dish 1 of Control Group 3 after 24 h of incubation; (B) Dish 1 of Experimental Group 3 after 24 h of incubation; (C) Dish 1 of Control Group 3 after 48 h of incubation; (D) Dish 1 of Experimental Group 3 after 48 h of incubation.
Visually, it is possible to notice that the colonies of Control Group 3 (see Figure 2A) presented a slightly larger diameter compared to those of Experimental Group 3 (Figure 2 B) after 24 hours of incubation. The diameters of non-exposed colonies (1.385 ± 0.32 mm) compared to those exposed (1.107 ± 0.39 mm) showed a significant difference in size (p = 0.042), which would be an indication that the colonies of Experimental Group 3 (although numerous) were affected by the fluence used. However, upon being incubated for another 24 hours, the surviving colonies continued to proliferate. In Figure 2 C and D, after 48 hours of incubation, the diameters of the colonies of Control Group 3 (2.35 ± 0.47 mm) showed no significant difference in comparison with those of Experimental Group 3 (2.27 ± 0.49 mm; p = 0.676).
4. Discussion
Photobiomodulation using blue light has been largely discussed as an alternative antimicrobial therapy. It shows positive results on in vitro experiments and demonstrates advantages as a treatment when compared with photodynamic therapy and ultraviolet light (Guffey and Wilborn, 2006GUFFEY, J.S. and WILBORN, J., 2006. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomedicine and Laser Surgery, vol. 24, no. 6, pp. 684-688. http://dx.doi.org/10.1089/pho.2006.24.684. PMid:17199466.
http://dx.doi.org/10.1089/pho.2006.24.68...
; Enwemeka et al., 2008ENWEMEKA, C.S., WILLIAMS, D., HOLLOSI, S., YENS, D. and ENWEMEKA, S.K., 2008. Visible 405 nm SLD light photo-destroys Methicilin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers in Surgery and Medicine, vol. 40, no. 10, pp. 734-737. http://dx.doi.org/10.1002/lsm.20724. PMid:19065556.
http://dx.doi.org/10.1002/lsm.20724...
, 2009ENWEMEKA, C.S., WILLIAMS, D., ENWEMEKA, S.K., HOLLOSI, S. and YENS, D., 2009. Blue 470-nm light kills Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomedicine and Laser Surgery, vol. 27, no. 2, pp. 221-226. http://dx.doi.org/10.1089/pho.2008.2413. PMid:19196103.
http://dx.doi.org/10.1089/pho.2008.2413...
; Chui et al., 2012CHUI, C., HIRATSUKA, K., AOKI, A., TAKEUCHI, Y., ABIKO, Y. and IZUMI, Y., 2012. Blue LED inhibits the growth of Porphyromonas gingivalis by suppressing the expression of genes associated with DNA replication and cell division. Lasers in Surgery and Medicine, vol. 44, no. 10, pp. 856-864. http://dx.doi.org/10.1002/lsm.22090. PMid:23129157.
http://dx.doi.org/10.1002/lsm.22090...
; De Lucca et al., 2012DE LUCCA, A.J., CARTER-WIENTJES, C., WILLIAMS, K.A. and BHATNAGAR, D., 2012. Blue light (470 nm) effectively inhibits bacterial and fungal growth. Letters in Applied Microbiology, vol. 55, no. 6, pp. 460-466. http://dx.doi.org/10.1111/lam.12002. PMid:23009190.
http://dx.doi.org/10.1111/lam.12002...
; Bumah et al., 2013BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S.E. and ENWEMEKA, C.S., 2013. Wavelength and bacterial density influence the bactericidal effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA). Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 547-553. http://dx.doi.org/10.1089/pho.2012.3461. PMid:23621894.
http://dx.doi.org/10.1089/pho.2012.3461...
, 2015BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S. and ENWEMEKA, C.S., 2015. Optimization of the antimicrobial effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers in Surgery and Medicine, vol. 47, no. 3, pp. 266-272. http://dx.doi.org/10.1002/lsm.22327. PMid:25639752.
http://dx.doi.org/10.1002/lsm.22327...
; Dai et al., 2013DAI, T., GUPTA, A., HUANG, Y.Y., SHERWOOD, M.E., MURRAY, C.K., VRAHAS, M.S., KIELIAN, T. and HAMBLIN, M.R., 2013. Blue light eliminates community-acquired Methicillin-resistant Staphylococcus aureus in infected mouse skin abrasions. Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 531-538. http://dx.doi.org/10.1089/pho.2012.3365. PMid:23406384.
http://dx.doi.org/10.1089/pho.2012.3365...
; Kim et al., 2013KIM, S.W., KIM, J.S., LIM, W.B., JEON, S.M., KIM, O.S., KOH, J.T., KIM, C.S., CHOI, H.R. and KIM, O.J., 2013. In vitro bactericidal effects of 625, 525, and 425 nm wavelength (red, green, and blue) light-emitting diode irradiation. Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 554-562. http://dx.doi.org/10.1089/pho.2012.3343. PMid:24138193.
http://dx.doi.org/10.1089/pho.2012.3343...
; Pileggi et al., 2013PILEGGI, G., WATAHA, J.C., GIRARD, M., GRAD, I., SCHRENZEL, J., LANGE, N. and BOUILLAGUET, S., 2013. Blue light-mediated inactivation of Enterococcus faecalis in vitro. Photodiagnosis and Photodynamic Therapy, vol. 10, no. 2, pp. 134-140. http://dx.doi.org/10.1016/j.pdpdt.2012.11.002. PMid:23769279.
http://dx.doi.org/10.1016/j.pdpdt.2012.1...
; Sousa et al., 2015SOUSA, N.T.A., SANTOS, M.F., GOMES, R.C., BRANDINO, H.E., MARTINEZ, R. and GUIRRO, R.R.J., 2015. Blue laser inhibits bacterial growth of Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. Photomedicine and Laser Surgery, vol. 33, no. 5, pp. 278-282. http://dx.doi.org/10.1089/pho.2014.3854. PMid:25954830.
http://dx.doi.org/10.1089/pho.2014.3854...
; Masson-Meyers et al., 2015MASSON-MEYERS, D.S., BUMAH, V.V., BIENER, G., RAICU, V. and ENWEMEKA, C.S., 2015. The relative antimicrobial effect of blue 405 nm LED and blue 405 nm laser on methicillin-resistant Staphylococcus aureus in vitro. Lasers in Medical Science, vol. 30, no. 9, pp. 2265-2271. http://dx.doi.org/10.1007/s10103-015-1799-1. PMid:26362142.
http://dx.doi.org/10.1007/s10103-015-179...
; Rosa et al., 2016ROSA, L.P., SILVA, F.C., VIANA, M.S. and MEIRA, G.A., 2016. In vitro effectiveness of 455-nm blue LED to reduce the load of Staphylococcus aureus and Candida albicans biofilms in compact bone tissue. Lasers in Medical Science, vol. 31, no. 1, pp. 27-32. http://dx.doi.org/10.1007/s10103-015-1826-2. PMid:26498601.
http://dx.doi.org/10.1007/s10103-015-182...
; Ferrer-Espada et al., 2019FERRER-ESPADA, R., WANG, Y., GOH, X.S. and DAI, T., 2019. Antimicrobial blue light inactivation of microbial isolates in biofilms. Lasers in Surgery and Medicine, In press. http://dx.doi.org/10.1002/lsm.23159. PMid:31536154.
http://dx.doi.org/10.1002/lsm.23159...
; Meurer et al., 2019MEURER, L., PAYNE, W. and GUFFEY, J.S., 2019. Visible light as an inhibitor of Camplyobacter jejuni. International Journal of Antimicrobial Agents, vol. 55, no. 1, pp. 105818. PMid:31593764.; Rupel et al., 2019RUPEL, K., ZUPIN, L., OTTAVIANI, G., BERTANI, I., MARTINELLI, V., PORRELLI, D., VODRET, S., VUERICH, R., PASSOS DA SILVA, D., BUSSANI, R., CROVELLA, S., PARSEK, M., VENTURI, V., DI LENARDA, R., BIASOTTO, M. and ZACCHIGNA, S., 2019. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. Biofilms and Microbiomes, vol. 5, no. 29, pp. 1-11. http://dx.doi.org/10.1038/s41522-019-0102-9. PMid:31602310.
http://dx.doi.org/10.1038/s41522-019-010...
; Schmid et al., 2019SCHMID, J., HOENES, K., VATTER, P. and HESSLING, M., 2019. Antimicrobial effect of visible light-photoinactivation of Legionella rubrilucens by irradiation at 450, 470, and 620 nm. Antibiotics (Basel, Switzerland), vol. 8, no. 187, pp. pii:E187. http://dx.doi.org/10.3390/antibiotics8040187. PMid:31618994.
http://dx.doi.org/10.3390/antibiotics804...
). Nevertheless, the great variety of experimental protocols using blue light on bacterial inhibition creates difficulty to identify ideal light emission parameters to be adopted with confidence.
Some investigations using blue light have reported 100% suppression of S. aureus proliferation on in vitro cultures (Guffey and Wilborn, 2006GUFFEY, J.S. and WILBORN, J., 2006. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomedicine and Laser Surgery, vol. 24, no. 6, pp. 684-688. http://dx.doi.org/10.1089/pho.2006.24.684. PMid:17199466.
http://dx.doi.org/10.1089/pho.2006.24.68...
; Bumah et al., 2015BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S. and ENWEMEKA, C.S., 2015. Optimization of the antimicrobial effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers in Surgery and Medicine, vol. 47, no. 3, pp. 266-272. http://dx.doi.org/10.1002/lsm.22327. PMid:25639752.
http://dx.doi.org/10.1002/lsm.22327...
). However, as shown in the present work, a great number of experiments described in the literature indicate only relative suppression of in vitro cultures when exposed to different patterns of the blue light emission (Enwemeka et al., 2009ENWEMEKA, C.S., WILLIAMS, D., ENWEMEKA, S.K., HOLLOSI, S. and YENS, D., 2009. Blue 470-nm light kills Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomedicine and Laser Surgery, vol. 27, no. 2, pp. 221-226. http://dx.doi.org/10.1089/pho.2008.2413. PMid:19196103.
http://dx.doi.org/10.1089/pho.2008.2413...
; Chebath-Taub et al., 2012CHEBATH-TAUB, D., STEINBERG, D., FEATHERSTONE, J.D.B. and FEUERSTEIN, O., 2012. Influence of blue light on Streptococcus mutans re-organization in biofilm. Journal of Photochemistry and Photobiology. B, Biology, vol. 116, pp. 75-78. http://dx.doi.org/10.1016/j.jphotobiol.2012.08.004. PMid:22982208.
http://dx.doi.org/10.1016/j.jphotobiol.2...
; Chui et al., 2012CHUI, C., HIRATSUKA, K., AOKI, A., TAKEUCHI, Y., ABIKO, Y. and IZUMI, Y., 2012. Blue LED inhibits the growth of Porphyromonas gingivalis by suppressing the expression of genes associated with DNA replication and cell division. Lasers in Surgery and Medicine, vol. 44, no. 10, pp. 856-864. http://dx.doi.org/10.1002/lsm.22090. PMid:23129157.
http://dx.doi.org/10.1002/lsm.22090...
; Dai et al., 2013DAI, T., GUPTA, A., HUANG, Y.Y., SHERWOOD, M.E., MURRAY, C.K., VRAHAS, M.S., KIELIAN, T. and HAMBLIN, M.R., 2013. Blue light eliminates community-acquired Methicillin-resistant Staphylococcus aureus in infected mouse skin abrasions. Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 531-538. http://dx.doi.org/10.1089/pho.2012.3365. PMid:23406384.
http://dx.doi.org/10.1089/pho.2012.3365...
; Guffey et al., 2013GUFFEY, J.S., PAYNE, W., JONES, T. and MARTIN, K., 2013. Evidence of resistance development by Staphylococcus aureus to an in vitro, multiple stage application of 405 nm light from a supraluminous diode array. Photomedicine and Laser Surgery, vol. 31, no. 4, pp. 179-182. http://dx.doi.org/10.1089/pho.2012.3450. PMid:23484587.
http://dx.doi.org/10.1089/pho.2012.3450...
; Kim et al., 2013KIM, S.W., KIM, J.S., LIM, W.B., JEON, S.M., KIM, O.S., KOH, J.T., KIM, C.S., CHOI, H.R. and KIM, O.J., 2013. In vitro bactericidal effects of 625, 525, and 425 nm wavelength (red, green, and blue) light-emitting diode irradiation. Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 554-562. http://dx.doi.org/10.1089/pho.2012.3343. PMid:24138193.
http://dx.doi.org/10.1089/pho.2012.3343...
; Sousa et al., 2015SOUSA, N.T.A., SANTOS, M.F., GOMES, R.C., BRANDINO, H.E., MARTINEZ, R. and GUIRRO, R.R.J., 2015. Blue laser inhibits bacterial growth of Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. Photomedicine and Laser Surgery, vol. 33, no. 5, pp. 278-282. http://dx.doi.org/10.1089/pho.2014.3854. PMid:25954830.
http://dx.doi.org/10.1089/pho.2014.3854...
; Masson-Meyers et al., 2015MASSON-MEYERS, D.S., BUMAH, V.V., BIENER, G., RAICU, V. and ENWEMEKA, C.S., 2015. The relative antimicrobial effect of blue 405 nm LED and blue 405 nm laser on methicillin-resistant Staphylococcus aureus in vitro. Lasers in Medical Science, vol. 30, no. 9, pp. 2265-2271. http://dx.doi.org/10.1007/s10103-015-1799-1. PMid:26362142.
http://dx.doi.org/10.1007/s10103-015-179...
; Ferrer-Espada et al., 2019FERRER-ESPADA, R., WANG, Y., GOH, X.S. and DAI, T., 2019. Antimicrobial blue light inactivation of microbial isolates in biofilms. Lasers in Surgery and Medicine, In press. http://dx.doi.org/10.1002/lsm.23159. PMid:31536154.
http://dx.doi.org/10.1002/lsm.23159...
; Rupel et al., 2019RUPEL, K., ZUPIN, L., OTTAVIANI, G., BERTANI, I., MARTINELLI, V., PORRELLI, D., VODRET, S., VUERICH, R., PASSOS DA SILVA, D., BUSSANI, R., CROVELLA, S., PARSEK, M., VENTURI, V., DI LENARDA, R., BIASOTTO, M. and ZACCHIGNA, S., 2019. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. Biofilms and Microbiomes, vol. 5, no. 29, pp. 1-11. http://dx.doi.org/10.1038/s41522-019-0102-9. PMid:31602310.
http://dx.doi.org/10.1038/s41522-019-010...
; Schmid et al., 2019SCHMID, J., HOENES, K., VATTER, P. and HESSLING, M., 2019. Antimicrobial effect of visible light-photoinactivation of Legionella rubrilucens by irradiation at 450, 470, and 620 nm. Antibiotics (Basel, Switzerland), vol. 8, no. 187, pp. pii:E187. http://dx.doi.org/10.3390/antibiotics8040187. PMid:31618994.
http://dx.doi.org/10.3390/antibiotics804...
). This fact demonstrates the influence of the pattern of exposure and the need for better standardization of optimal parameters to obtain the best effect.
Some studies suggest that the bacterial inhibition action of blue light exposure is related to an increase of reactive oxygen species (ROS) that mediates harmful actions to the cell (Guffey and Wilborn, 2006GUFFEY, J.S. and WILBORN, J., 2006. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomedicine and Laser Surgery, vol. 24, no. 6, pp. 684-688. http://dx.doi.org/10.1089/pho.2006.24.684. PMid:17199466.
http://dx.doi.org/10.1089/pho.2006.24.68...
; Enwemeka et al., 2008ENWEMEKA, C.S., WILLIAMS, D., HOLLOSI, S., YENS, D. and ENWEMEKA, S.K., 2008. Visible 405 nm SLD light photo-destroys Methicilin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers in Surgery and Medicine, vol. 40, no. 10, pp. 734-737. http://dx.doi.org/10.1002/lsm.20724. PMid:19065556.
http://dx.doi.org/10.1002/lsm.20724...
; Lipovsky et al., 2010LIPOVSKY, A., NITZAN, Y., GEDANKEN, A. and LUBART, R., 2010. Visible light-induced killing of bacteria as a function of wavelength: implication for wound healing. Lasers in Surgery and Medicine, vol. 42, no. 6, pp. 467-472. http://dx.doi.org/10.1002/lsm.20948. PMid:20662022.
http://dx.doi.org/10.1002/lsm.20948...
; Dai et al., 2012DAI, T., GUPTA, A., MURRAY, C.K., VRAHAS, M.S., TEGOS, G.P. and HAMBLIN, M.R., 2012. Blue light for infectious diseases: propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resistance Updates, vol. 15, no. 4, pp. 223-236. http://dx.doi.org/10.1016/j.drup.2012.07.001. PMid:22846406.
http://dx.doi.org/10.1016/j.drup.2012.07...
; Chui et al., 2012CHUI, C., HIRATSUKA, K., AOKI, A., TAKEUCHI, Y., ABIKO, Y. and IZUMI, Y., 2012. Blue LED inhibits the growth of Porphyromonas gingivalis by suppressing the expression of genes associated with DNA replication and cell division. Lasers in Surgery and Medicine, vol. 44, no. 10, pp. 856-864. http://dx.doi.org/10.1002/lsm.22090. PMid:23129157.
http://dx.doi.org/10.1002/lsm.22090...
; Kim et al., 2013KIM, S.W., KIM, J.S., LIM, W.B., JEON, S.M., KIM, O.S., KOH, J.T., KIM, C.S., CHOI, H.R. and KIM, O.J., 2013. In vitro bactericidal effects of 625, 525, and 425 nm wavelength (red, green, and blue) light-emitting diode irradiation. Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 554-562. http://dx.doi.org/10.1089/pho.2012.3343. PMid:24138193.
http://dx.doi.org/10.1089/pho.2012.3343...
; Bumah et al., 2015BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S. and ENWEMEKA, C.S., 2015. Optimization of the antimicrobial effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers in Surgery and Medicine, vol. 47, no. 3, pp. 266-272. http://dx.doi.org/10.1002/lsm.22327. PMid:25639752.
http://dx.doi.org/10.1002/lsm.22327...
; Masson-Meyers et al., 2015MASSON-MEYERS, D.S., BUMAH, V.V., BIENER, G., RAICU, V. and ENWEMEKA, C.S., 2015. The relative antimicrobial effect of blue 405 nm LED and blue 405 nm laser on methicillin-resistant Staphylococcus aureus in vitro. Lasers in Medical Science, vol. 30, no. 9, pp. 2265-2271. http://dx.doi.org/10.1007/s10103-015-1799-1. PMid:26362142.
http://dx.doi.org/10.1007/s10103-015-179...
; Rupel et al., 2019RUPEL, K., ZUPIN, L., OTTAVIANI, G., BERTANI, I., MARTINELLI, V., PORRELLI, D., VODRET, S., VUERICH, R., PASSOS DA SILVA, D., BUSSANI, R., CROVELLA, S., PARSEK, M., VENTURI, V., DI LENARDA, R., BIASOTTO, M. and ZACCHIGNA, S., 2019. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. Biofilms and Microbiomes, vol. 5, no. 29, pp. 1-11. http://dx.doi.org/10.1038/s41522-019-0102-9. PMid:31602310.
http://dx.doi.org/10.1038/s41522-019-010...
). Mouse and human cells exposed to blue light generates ROS production at levels that were not observed when the same type of cells was exposed to red or infrared light (Kushibiki et al., 2013KUSHIBIKI, T., HIRASAWA, T., OKAWA, S. and ISHIHARA, M., 2013. Blue laser irradiation generates intracellular reactive oxygen species in various types of cells. Photomedicine and Laser Surgery, vol. 31, no. 3, pp. 95-104. http://dx.doi.org/10.1089/pho.2012.3361. PMid:23390956.
http://dx.doi.org/10.1089/pho.2012.3361...
). Some researchers indicate that even with high fluence values, the blue light does not harm DNA from mouse cells (Zhang et al., 2014ZHANG, Y., ZHU, Y., GUPTA, A., HUANG, Y., MURRAY, C.K., VRAHAS, M.S., SHERWOOD, M.E., BAER, D.G., HAMBLIN, M.R. and DAI, T., 2014. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: implications for prophylaxis and treatment of combat-related wound infections. The Journal of Infectious Diseases, vol. 209, no. 12, pp. 1963-1971. http://dx.doi.org/10.1093/infdis/jit842. PMid:24381206.
http://dx.doi.org/10.1093/infdis/jit842...
; Rupel et al., 2019RUPEL, K., ZUPIN, L., OTTAVIANI, G., BERTANI, I., MARTINELLI, V., PORRELLI, D., VODRET, S., VUERICH, R., PASSOS DA SILVA, D., BUSSANI, R., CROVELLA, S., PARSEK, M., VENTURI, V., DI LENARDA, R., BIASOTTO, M. and ZACCHIGNA, S., 2019. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. Biofilms and Microbiomes, vol. 5, no. 29, pp. 1-11. http://dx.doi.org/10.1038/s41522-019-0102-9. PMid:31602310.
http://dx.doi.org/10.1038/s41522-019-010...
). However, other reports indicate that 410-420 nm wavelength emissions could cause cytotoxic fluence-dependent effects on human fibroblasts (Opländer et al., 2011OPLÄNDER, C., HIDDING, S., WERNERS, F.B., BORN, M., PALLUA, N. and SUSCHEK, C.V., 2011. Effects of blue light irradiation on human dermal fibroblasts. Journal of Photochemistry and Photobiology. B, Biology, vol. 103, no. 2, pp. 118-125. http://dx.doi.org/10.1016/j.jphotobiol.2011.02.018. PMid:21421326.
http://dx.doi.org/10.1016/j.jphotobiol.2...
).
Therefore, it is necessary to define emission parameters that inhibit S. aureus without bringing damage to human cells. The first parameter that needs attention is the wavelength. Different values starting from 405 nm have been used to verify the possible effect against S. aureus (Guffey and Wilborn, 2006GUFFEY, J.S. and WILBORN, J., 2006. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomedicine and Laser Surgery, vol. 24, no. 6, pp. 684-688. http://dx.doi.org/10.1089/pho.2006.24.684. PMid:17199466.
http://dx.doi.org/10.1089/pho.2006.24.68...
; Lipovsky et al., 2010LIPOVSKY, A., NITZAN, Y., GEDANKEN, A. and LUBART, R., 2010. Visible light-induced killing of bacteria as a function of wavelength: implication for wound healing. Lasers in Surgery and Medicine, vol. 42, no. 6, pp. 467-472. http://dx.doi.org/10.1002/lsm.20948. PMid:20662022.
http://dx.doi.org/10.1002/lsm.20948...
; Bumah et al., 2013BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S.E. and ENWEMEKA, C.S., 2013. Wavelength and bacterial density influence the bactericidal effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA). Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 547-553. http://dx.doi.org/10.1089/pho.2012.3461. PMid:23621894.
http://dx.doi.org/10.1089/pho.2012.3461...
, 2015BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S. and ENWEMEKA, C.S., 2015. Optimization of the antimicrobial effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers in Surgery and Medicine, vol. 47, no. 3, pp. 266-272. http://dx.doi.org/10.1002/lsm.22327. PMid:25639752.
http://dx.doi.org/10.1002/lsm.22327...
). Values close to 470 nm are often chosen, showing good antimicrobial suppression results (Guffey and Wilborn, 2006GUFFEY, J.S. and WILBORN, J., 2006. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomedicine and Laser Surgery, vol. 24, no. 6, pp. 684-688. http://dx.doi.org/10.1089/pho.2006.24.684. PMid:17199466.
http://dx.doi.org/10.1089/pho.2006.24.68...
; Enwemeka et al., 2009ENWEMEKA, C.S., WILLIAMS, D., ENWEMEKA, S.K., HOLLOSI, S. and YENS, D., 2009. Blue 470-nm light kills Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomedicine and Laser Surgery, vol. 27, no. 2, pp. 221-226. http://dx.doi.org/10.1089/pho.2008.2413. PMid:19196103.
http://dx.doi.org/10.1089/pho.2008.2413...
; Dai et al., 2012DAI, T., GUPTA, A., MURRAY, C.K., VRAHAS, M.S., TEGOS, G.P. and HAMBLIN, M.R., 2012. Blue light for infectious diseases: propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resistance Updates, vol. 15, no. 4, pp. 223-236. http://dx.doi.org/10.1016/j.drup.2012.07.001. PMid:22846406.
http://dx.doi.org/10.1016/j.drup.2012.07...
; De Lucca et al., 2012DE LUCCA, A.J., CARTER-WIENTJES, C., WILLIAMS, K.A. and BHATNAGAR, D., 2012. Blue light (470 nm) effectively inhibits bacterial and fungal growth. Letters in Applied Microbiology, vol. 55, no. 6, pp. 460-466. http://dx.doi.org/10.1111/lam.12002. PMid:23009190.
http://dx.doi.org/10.1111/lam.12002...
; Bumah et al., 2013BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S.E. and ENWEMEKA, C.S., 2013. Wavelength and bacterial density influence the bactericidal effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA). Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 547-553. http://dx.doi.org/10.1089/pho.2012.3461. PMid:23621894.
http://dx.doi.org/10.1089/pho.2012.3461...
, 2015BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S. and ENWEMEKA, C.S., 2015. Optimization of the antimicrobial effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers in Surgery and Medicine, vol. 47, no. 3, pp. 266-272. http://dx.doi.org/10.1002/lsm.22327. PMid:25639752.
http://dx.doi.org/10.1002/lsm.22327...
; Pileggi et al., 2013PILEGGI, G., WATAHA, J.C., GIRARD, M., GRAD, I., SCHRENZEL, J., LANGE, N. and BOUILLAGUET, S., 2013. Blue light-mediated inactivation of Enterococcus faecalis in vitro. Photodiagnosis and Photodynamic Therapy, vol. 10, no. 2, pp. 134-140. http://dx.doi.org/10.1016/j.pdpdt.2012.11.002. PMid:23769279.
http://dx.doi.org/10.1016/j.pdpdt.2012.1...
; Sousa et al., 2015SOUSA, N.T.A., SANTOS, M.F., GOMES, R.C., BRANDINO, H.E., MARTINEZ, R. and GUIRRO, R.R.J., 2015. Blue laser inhibits bacterial growth of Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. Photomedicine and Laser Surgery, vol. 33, no. 5, pp. 278-282. http://dx.doi.org/10.1089/pho.2014.3854. PMid:25954830.
http://dx.doi.org/10.1089/pho.2014.3854...
; Rosa et al., 2016ROSA, L.P., SILVA, F.C., VIANA, M.S. and MEIRA, G.A., 2016. In vitro effectiveness of 455-nm blue LED to reduce the load of Staphylococcus aureus and Candida albicans biofilms in compact bone tissue. Lasers in Medical Science, vol. 31, no. 1, pp. 27-32. http://dx.doi.org/10.1007/s10103-015-1826-2. PMid:26498601.
http://dx.doi.org/10.1007/s10103-015-182...
; Cohen-Berneron et al., 2016COHEN-BERNERON, J., STEINBERG, D., FEATHERSTONE, J.D.B. and FEUERSTEIN, O., 2016. Sustained effects of blue light on Streptococcus mutans in regrown biofilm. Lasers in Medical Science, vol. 31, no. 3, pp. 445-452. http://dx.doi.org/10.1007/s10103-016-1873-3. PMid:26796707.
http://dx.doi.org/10.1007/s10103-016-187...
; Ferrer-Espada et al., 2019FERRER-ESPADA, R., WANG, Y., GOH, X.S. and DAI, T., 2019. Antimicrobial blue light inactivation of microbial isolates in biofilms. Lasers in Surgery and Medicine, In press. http://dx.doi.org/10.1002/lsm.23159. PMid:31536154.
http://dx.doi.org/10.1002/lsm.23159...
; Meurer et al., 2019MEURER, L., PAYNE, W. and GUFFEY, J.S., 2019. Visible light as an inhibitor of Camplyobacter jejuni. International Journal of Antimicrobial Agents, vol. 55, no. 1, pp. 105818. PMid:31593764.; Rupel et al., 2019RUPEL, K., ZUPIN, L., OTTAVIANI, G., BERTANI, I., MARTINELLI, V., PORRELLI, D., VODRET, S., VUERICH, R., PASSOS DA SILVA, D., BUSSANI, R., CROVELLA, S., PARSEK, M., VENTURI, V., DI LENARDA, R., BIASOTTO, M. and ZACCHIGNA, S., 2019. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. Biofilms and Microbiomes, vol. 5, no. 29, pp. 1-11. http://dx.doi.org/10.1038/s41522-019-0102-9. PMid:31602310.
http://dx.doi.org/10.1038/s41522-019-010...
; Schmid et al., 2019SCHMID, J., HOENES, K., VATTER, P. and HESSLING, M., 2019. Antimicrobial effect of visible light-photoinactivation of Legionella rubrilucens by irradiation at 450, 470, and 620 nm. Antibiotics (Basel, Switzerland), vol. 8, no. 187, pp. pii:E187. http://dx.doi.org/10.3390/antibiotics8040187. PMid:31618994.
http://dx.doi.org/10.3390/antibiotics804...
). Moreover, it has been shown that values between 453 and 480 nm are less cytotoxic to human cells (Opländer et al., 2011OPLÄNDER, C., HIDDING, S., WERNERS, F.B., BORN, M., PALLUA, N. and SUSCHEK, C.V., 2011. Effects of blue light irradiation on human dermal fibroblasts. Journal of Photochemistry and Photobiology. B, Biology, vol. 103, no. 2, pp. 118-125. http://dx.doi.org/10.1016/j.jphotobiol.2011.02.018. PMid:21421326.
http://dx.doi.org/10.1016/j.jphotobiol.2...
; Rupel et al., 2019RUPEL, K., ZUPIN, L., OTTAVIANI, G., BERTANI, I., MARTINELLI, V., PORRELLI, D., VODRET, S., VUERICH, R., PASSOS DA SILVA, D., BUSSANI, R., CROVELLA, S., PARSEK, M., VENTURI, V., DI LENARDA, R., BIASOTTO, M. and ZACCHIGNA, S., 2019. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. Biofilms and Microbiomes, vol. 5, no. 29, pp. 1-11. http://dx.doi.org/10.1038/s41522-019-0102-9. PMid:31602310.
http://dx.doi.org/10.1038/s41522-019-010...
). This suggests that values on this range (including the one used on the present paper) may be used as a pattern against S. aureus, due to its antimicrobial efficacy and greater safety level to host cells.
Irradiance represents the radiation flow per area unity received by a surface (Hadis et al., 2016HADIS, M.A., ZAINAL, S.A., HOLDER, M.S., CARROLL, J.D., COOPER, P.R., MILWARD, M.R. and PALIN, W.M., 2016. The dark art of light measurement: accurate radiometry for low-level light therapy. Lasers in Medical Science, vol. 31, no. 4, pp. 789-809. http://dx.doi.org/10.1007/s10103-016-1914-y. PMid:26964800.
http://dx.doi.org/10.1007/s10103-016-191...
), it is a variable parameter in different researches, although, in general, the values used are classified as photoemission of low intensity (Huang et al., 2011HUANG, Y.Y., SHARMA, S.K., CARROLL, J. and HAMBLIN, M.R., 2011. Biphasic dose response in low level light therapy - an update. Dose-Response, vol. 9, no. 4, pp. 602-618. http://dx.doi.org/10.2203/dose-response.11-009.Hamblin. PMid:22461763.
http://dx.doi.org/10.2203/dose-response....
; Farivar et al., 2014FARIVAR, S., MALEKSHAHABI, T. and SHIARI, R., 2014. Biological effects of low level laser therapy. Journal of Lasers in Medical Sciences, vol. 5, no. 2, pp. 58-62. PMid:25653800.). The protocols adopted in some investigations use 70 to 610 mW/cm (Enwemeka et al., 2008ENWEMEKA, C.S., WILLIAMS, D., HOLLOSI, S., YENS, D. and ENWEMEKA, S.K., 2008. Visible 405 nm SLD light photo-destroys Methicilin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers in Surgery and Medicine, vol. 40, no. 10, pp. 734-737. http://dx.doi.org/10.1002/lsm.20724. PMid:19065556.
http://dx.doi.org/10.1002/lsm.20724...
; Lipovsky et al., 2010LIPOVSKY, A., NITZAN, Y., GEDANKEN, A. and LUBART, R., 2010. Visible light-induced killing of bacteria as a function of wavelength: implication for wound healing. Lasers in Surgery and Medicine, vol. 42, no. 6, pp. 467-472. http://dx.doi.org/10.1002/lsm.20948. PMid:20662022.
http://dx.doi.org/10.1002/lsm.20948...
; Bumah et al., 2013BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S.E. and ENWEMEKA, C.S., 2013. Wavelength and bacterial density influence the bactericidal effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA). Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 547-553. http://dx.doi.org/10.1089/pho.2012.3461. PMid:23621894.
http://dx.doi.org/10.1089/pho.2012.3461...
; Sousa et al., 2015SOUSA, N.T.A., SANTOS, M.F., GOMES, R.C., BRANDINO, H.E., MARTINEZ, R. and GUIRRO, R.R.J., 2015. Blue laser inhibits bacterial growth of Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. Photomedicine and Laser Surgery, vol. 33, no. 5, pp. 278-282. http://dx.doi.org/10.1089/pho.2014.3854. PMid:25954830.
http://dx.doi.org/10.1089/pho.2014.3854...
; Masson-Meyers et al., 2015MASSON-MEYERS, D.S., BUMAH, V.V., BIENER, G., RAICU, V. and ENWEMEKA, C.S., 2015. The relative antimicrobial effect of blue 405 nm LED and blue 405 nm laser on methicillin-resistant Staphylococcus aureus in vitro. Lasers in Medical Science, vol. 30, no. 9, pp. 2265-2271. http://dx.doi.org/10.1007/s10103-015-1799-1. PMid:26362142.
http://dx.doi.org/10.1007/s10103-015-179...
; Rosa et al., 2016ROSA, L.P., SILVA, F.C., VIANA, M.S. and MEIRA, G.A., 2016. In vitro effectiveness of 455-nm blue LED to reduce the load of Staphylococcus aureus and Candida albicans biofilms in compact bone tissue. Lasers in Medical Science, vol. 31, no. 1, pp. 27-32. http://dx.doi.org/10.1007/s10103-015-1826-2. PMid:26498601.
http://dx.doi.org/10.1007/s10103-015-182...
; Rupel et al., 2019RUPEL, K., ZUPIN, L., OTTAVIANI, G., BERTANI, I., MARTINELLI, V., PORRELLI, D., VODRET, S., VUERICH, R., PASSOS DA SILVA, D., BUSSANI, R., CROVELLA, S., PARSEK, M., VENTURI, V., DI LENARDA, R., BIASOTTO, M. and ZACCHIGNA, S., 2019. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. Biofilms and Microbiomes, vol. 5, no. 29, pp. 1-11. http://dx.doi.org/10.1038/s41522-019-0102-9. PMid:31602310.
http://dx.doi.org/10.1038/s41522-019-010...
), in other ones, values varies from 10 to 30 mW/cm (Enwemeka et al., 2009ENWEMEKA, C.S., WILLIAMS, D., ENWEMEKA, S.K., HOLLOSI, S. and YENS, D., 2009. Blue 470-nm light kills Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomedicine and Laser Surgery, vol. 27, no. 2, pp. 221-226. http://dx.doi.org/10.1089/pho.2008.2413. PMid:19196103.
http://dx.doi.org/10.1089/pho.2008.2413...
; Bumah et al., 2013BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S.E. and ENWEMEKA, C.S., 2013. Wavelength and bacterial density influence the bactericidal effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA). Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 547-553. http://dx.doi.org/10.1089/pho.2012.3461. PMid:23621894.
http://dx.doi.org/10.1089/pho.2012.3461...
; Dai et al., 2013DAI, T., GUPTA, A., HUANG, Y.Y., SHERWOOD, M.E., MURRAY, C.K., VRAHAS, M.S., KIELIAN, T. and HAMBLIN, M.R., 2013. Blue light eliminates community-acquired Methicillin-resistant Staphylococcus aureus in infected mouse skin abrasions. Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 531-538. http://dx.doi.org/10.1089/pho.2012.3365. PMid:23406384.
http://dx.doi.org/10.1089/pho.2012.3365...
; Kim et al., 2013KIM, S.W., KIM, J.S., LIM, W.B., JEON, S.M., KIM, O.S., KOH, J.T., KIM, C.S., CHOI, H.R. and KIM, O.J., 2013. In vitro bactericidal effects of 625, 525, and 425 nm wavelength (red, green, and blue) light-emitting diode irradiation. Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 554-562. http://dx.doi.org/10.1089/pho.2012.3343. PMid:24138193.
http://dx.doi.org/10.1089/pho.2012.3343...
; Zhang et al., 2014ZHANG, Y., ZHU, Y., GUPTA, A., HUANG, Y., MURRAY, C.K., VRAHAS, M.S., SHERWOOD, M.E., BAER, D.G., HAMBLIN, M.R. and DAI, T., 2014. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: implications for prophylaxis and treatment of combat-related wound infections. The Journal of Infectious Diseases, vol. 209, no. 12, pp. 1963-1971. http://dx.doi.org/10.1093/infdis/jit842. PMid:24381206.
http://dx.doi.org/10.1093/infdis/jit842...
; Schmid et al., 2019SCHMID, J., HOENES, K., VATTER, P. and HESSLING, M., 2019. Antimicrobial effect of visible light-photoinactivation of Legionella rubrilucens by irradiation at 450, 470, and 620 nm. Antibiotics (Basel, Switzerland), vol. 8, no. 187, pp. pii:E187. http://dx.doi.org/10.3390/antibiotics8040187. PMid:31618994.
http://dx.doi.org/10.3390/antibiotics804...
). However, it is difficult to establish a relation between low intensity irradiation values and bacterial inhibitory effect, because the results show similar inhibition levels. It is important to highlight that high irradiance patterns can extrapolate the limit of what is considered low intensity light irradiation (Farivar et al., 2014FARIVAR, S., MALEKSHAHABI, T. and SHIARI, R., 2014. Biological effects of low level laser therapy. Journal of Lasers in Medical Sciences, vol. 5, no. 2, pp. 58-62. PMid:25653800.). On the other hand, when using very low values of this parameter, the light emission might last for too much time. The irradiances used in the present research (19.92 and 35.22 mW/cm2) showed results for in vitro experiments, although, it might not be the best option for in vivo experiments (considering the time spent), reinforcing that ≥70 mW/cm2 may be the best interval for the irradiance choice.
Fluence is the energy delivered per cell area during a phototherapeutic emission (Hadis et al., 2016HADIS, M.A., ZAINAL, S.A., HOLDER, M.S., CARROLL, J.D., COOPER, P.R., MILWARD, M.R. and PALIN, W.M., 2016. The dark art of light measurement: accurate radiometry for low-level light therapy. Lasers in Medical Science, vol. 31, no. 4, pp. 789-809. http://dx.doi.org/10.1007/s10103-016-1914-y. PMid:26964800.
http://dx.doi.org/10.1007/s10103-016-191...
). This is an important parameter when studying blue light antimicrobial effects, also the one that presents the higher discrepancy on protocols used in different experiments. Some researchers state that only higher values (above 100 J/cm2) are effective against S. aureus (Lipovsky et al., 2010LIPOVSKY, A., NITZAN, Y., GEDANKEN, A. and LUBART, R., 2010. Visible light-induced killing of bacteria as a function of wavelength: implication for wound healing. Lasers in Surgery and Medicine, vol. 42, no. 6, pp. 467-472. http://dx.doi.org/10.1002/lsm.20948. PMid:20662022.
http://dx.doi.org/10.1002/lsm.20948...
; Chui et al., 2012CHUI, C., HIRATSUKA, K., AOKI, A., TAKEUCHI, Y., ABIKO, Y. and IZUMI, Y., 2012. Blue LED inhibits the growth of Porphyromonas gingivalis by suppressing the expression of genes associated with DNA replication and cell division. Lasers in Surgery and Medicine, vol. 44, no. 10, pp. 856-864. http://dx.doi.org/10.1002/lsm.22090. PMid:23129157.
http://dx.doi.org/10.1002/lsm.22090...
; Ferrer-Espada et al., 2019FERRER-ESPADA, R., WANG, Y., GOH, X.S. and DAI, T., 2019. Antimicrobial blue light inactivation of microbial isolates in biofilms. Lasers in Surgery and Medicine, In press. http://dx.doi.org/10.1002/lsm.23159. PMid:31536154.
http://dx.doi.org/10.1002/lsm.23159...
); besides that, the supposed fluence-dependent inhibition effect cause controversial among scientific community (Guffey and Wilborn, 2006GUFFEY, J.S. and WILBORN, J., 2006. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomedicine and Laser Surgery, vol. 24, no. 6, pp. 684-688. http://dx.doi.org/10.1089/pho.2006.24.684. PMid:17199466.
http://dx.doi.org/10.1089/pho.2006.24.68...
; Enwemeka et al., 2009ENWEMEKA, C.S., WILLIAMS, D., ENWEMEKA, S.K., HOLLOSI, S. and YENS, D., 2009. Blue 470-nm light kills Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomedicine and Laser Surgery, vol. 27, no. 2, pp. 221-226. http://dx.doi.org/10.1089/pho.2008.2413. PMid:19196103.
http://dx.doi.org/10.1089/pho.2008.2413...
; Lipovsky et al., 2010LIPOVSKY, A., NITZAN, Y., GEDANKEN, A. and LUBART, R., 2010. Visible light-induced killing of bacteria as a function of wavelength: implication for wound healing. Lasers in Surgery and Medicine, vol. 42, no. 6, pp. 467-472. http://dx.doi.org/10.1002/lsm.20948. PMid:20662022.
http://dx.doi.org/10.1002/lsm.20948...
; Sousa et al., 2015SOUSA, N.T.A., SANTOS, M.F., GOMES, R.C., BRANDINO, H.E., MARTINEZ, R. and GUIRRO, R.R.J., 2015. Blue laser inhibits bacterial growth of Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. Photomedicine and Laser Surgery, vol. 33, no. 5, pp. 278-282. http://dx.doi.org/10.1089/pho.2014.3854. PMid:25954830.
http://dx.doi.org/10.1089/pho.2014.3854...
; Rupel et al., 2019RUPEL, K., ZUPIN, L., OTTAVIANI, G., BERTANI, I., MARTINELLI, V., PORRELLI, D., VODRET, S., VUERICH, R., PASSOS DA SILVA, D., BUSSANI, R., CROVELLA, S., PARSEK, M., VENTURI, V., DI LENARDA, R., BIASOTTO, M. and ZACCHIGNA, S., 2019. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. Biofilms and Microbiomes, vol. 5, no. 29, pp. 1-11. http://dx.doi.org/10.1038/s41522-019-0102-9. PMid:31602310.
http://dx.doi.org/10.1038/s41522-019-010...
).
In this research, low fluencies were adopted: 16.29, 27.26 and 54.32 J/cm2. When single exposure was used, no inhibition was noticed on irradiated cultures. In addition, it has been reported that low fluencies and the unique application of blue light have the potential to generate bacterial resistance (Guffey et al., 2013GUFFEY, J.S., PAYNE, W., JONES, T. and MARTIN, K., 2013. Evidence of resistance development by Staphylococcus aureus to an in vitro, multiple stage application of 405 nm light from a supraluminous diode array. Photomedicine and Laser Surgery, vol. 31, no. 4, pp. 179-182. http://dx.doi.org/10.1089/pho.2012.3450. PMid:23484587.
http://dx.doi.org/10.1089/pho.2012.3450...
); thus, it is evident that this emission pattern is not desirable as an antibacterial method, representing a risk in hypothetical clinical use. However, when the exposure was fractionated in three applications, partial bacterial growth inhibition was verified. This result indicates that the fractionation of the blue light application optimizes its antimicrobial action on S. aureus. However, even when using fractional application, the use of low fluencies seems to be poorly recommended, as other authors have also been suggesting (Guffey et al., 2013GUFFEY, J.S., PAYNE, W., JONES, T. and MARTIN, K., 2013. Evidence of resistance development by Staphylococcus aureus to an in vitro, multiple stage application of 405 nm light from a supraluminous diode array. Photomedicine and Laser Surgery, vol. 31, no. 4, pp. 179-182. http://dx.doi.org/10.1089/pho.2012.3450. PMid:23484587.
http://dx.doi.org/10.1089/pho.2012.3450...
; Rosa et al., 2016ROSA, L.P., SILVA, F.C., VIANA, M.S. and MEIRA, G.A., 2016. In vitro effectiveness of 455-nm blue LED to reduce the load of Staphylococcus aureus and Candida albicans biofilms in compact bone tissue. Lasers in Medical Science, vol. 31, no. 1, pp. 27-32. http://dx.doi.org/10.1007/s10103-015-1826-2. PMid:26498601.
http://dx.doi.org/10.1007/s10103-015-182...
).
In the present study, the inhibition pattern found as a result of Experiment 1 indicates antibacterial effect on the superficial layers of the bacterial colony, however, it shows surviving bacteria proliferating in deeper layers of the colony.
In addition, Experiment 2 showed that there is no significant suppression of bacterial growth when 54.32 J/cm2 fluence was used, even in fractionated application as measured by colony counts. Furthermore, at the end of the experiment the proliferation of all bacterial colonies has not been disrupted, which shows that low fluencies of blue light probably are not even capable of bacteriostatic action. The limited size of colonies grown upon exposure to blue light suggests an important influence on the metabolism and/or replication mechanism of this microorganism, but at the low fluencies used, this apparent harmful action was limited and the bacteria was able to circumvent this threat. This indicates that, regardless of the mechanism of action, the bacterial inhibition generated by the blue light becomes effective only at higher dosages, and it is important to verify if the proposed mechanism of action justifies this phenomenon. Thus, the best way to use the blue light against S. aureus could be the adoption of higher fluence values and the use of fractionated exposure of the light emission. A recently published paper has addressed the importance of the fluence and irradiance on blue light effectiveness using Pseudomonas aeruginosa as subject of the study and demonstrated similar observations as shown here (Rupel et al., 2019RUPEL, K., ZUPIN, L., OTTAVIANI, G., BERTANI, I., MARTINELLI, V., PORRELLI, D., VODRET, S., VUERICH, R., PASSOS DA SILVA, D., BUSSANI, R., CROVELLA, S., PARSEK, M., VENTURI, V., DI LENARDA, R., BIASOTTO, M. and ZACCHIGNA, S., 2019. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. Biofilms and Microbiomes, vol. 5, no. 29, pp. 1-11. http://dx.doi.org/10.1038/s41522-019-0102-9. PMid:31602310.
http://dx.doi.org/10.1038/s41522-019-010...
).
Therefore, it is important to continue address the issues discussed here and focus on the determination of safe parameters of blue light emission that can be used as a powerful bactericidal agent while not damaging host tissues. Once this is done, it will be possible to conduct in vivo experiments with greater precision, in order to better justify its use in human beings.
5. Conclusion
The present study showed that the use of low fluencies of blue light, provided by Light-Emitting Diodes, is not enough to generate satisfactory suppression in cultures of Staphylococcus aureus. Although the fluence of 54.32 J/cm2 (fractionated in three applications) generated partial bacterial inhibition, the affected colonies were able to adapt and continue their proliferation. These results show the ineffectiveness of the use of low parameters values of blue light as an antimicrobial method since they promote only a reduction of bacterial growth and therefore would be of limited therapeutic potential.
Acknowledgements
We would like to thank CAPES - Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil, for the fellowship awarded to I. D. C. Galo.
-
(With 2 figures)
References
- BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S.E. and ENWEMEKA, C.S., 2013. Wavelength and bacterial density influence the bactericidal effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA). Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 547-553. http://dx.doi.org/10.1089/pho.2012.3461 PMid:23621894.
» http://dx.doi.org/10.1089/pho.2012.3461 - BUMAH, V.V., MASSON-MEYERS, D.S., CASHIN, S. and ENWEMEKA, C.S., 2015. Optimization of the antimicrobial effect of blue light on Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers in Surgery and Medicine, vol. 47, no. 3, pp. 266-272. http://dx.doi.org/10.1002/lsm.22327 PMid:25639752.
» http://dx.doi.org/10.1002/lsm.22327 - CHEBATH-TAUB, D., STEINBERG, D., FEATHERSTONE, J.D.B. and FEUERSTEIN, O., 2012. Influence of blue light on Streptococcus mutans re-organization in biofilm. Journal of Photochemistry and Photobiology. B, Biology, vol. 116, pp. 75-78. http://dx.doi.org/10.1016/j.jphotobiol.2012.08.004 PMid:22982208.
» http://dx.doi.org/10.1016/j.jphotobiol.2012.08.004 - CHUI, C., HIRATSUKA, K., AOKI, A., TAKEUCHI, Y., ABIKO, Y. and IZUMI, Y., 2012. Blue LED inhibits the growth of Porphyromonas gingivalis by suppressing the expression of genes associated with DNA replication and cell division. Lasers in Surgery and Medicine, vol. 44, no. 10, pp. 856-864. http://dx.doi.org/10.1002/lsm.22090 PMid:23129157.
» http://dx.doi.org/10.1002/lsm.22090 - COHEN-BERNERON, J., STEINBERG, D., FEATHERSTONE, J.D.B. and FEUERSTEIN, O., 2016. Sustained effects of blue light on Streptococcus mutans in regrown biofilm. Lasers in Medical Science, vol. 31, no. 3, pp. 445-452. http://dx.doi.org/10.1007/s10103-016-1873-3 PMid:26796707.
» http://dx.doi.org/10.1007/s10103-016-1873-3 - DAI, T., GUPTA, A., HUANG, Y.Y., SHERWOOD, M.E., MURRAY, C.K., VRAHAS, M.S., KIELIAN, T. and HAMBLIN, M.R., 2013. Blue light eliminates community-acquired Methicillin-resistant Staphylococcus aureus in infected mouse skin abrasions. Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 531-538. http://dx.doi.org/10.1089/pho.2012.3365 PMid:23406384.
» http://dx.doi.org/10.1089/pho.2012.3365 - DAI, T., GUPTA, A., MURRAY, C.K., VRAHAS, M.S., TEGOS, G.P. and HAMBLIN, M.R., 2012. Blue light for infectious diseases: propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resistance Updates, vol. 15, no. 4, pp. 223-236. http://dx.doi.org/10.1016/j.drup.2012.07.001 PMid:22846406.
» http://dx.doi.org/10.1016/j.drup.2012.07.001 - DE LUCCA, A.J., CARTER-WIENTJES, C., WILLIAMS, K.A. and BHATNAGAR, D., 2012. Blue light (470 nm) effectively inhibits bacterial and fungal growth. Letters in Applied Microbiology, vol. 55, no. 6, pp. 460-466. http://dx.doi.org/10.1111/lam.12002 PMid:23009190.
» http://dx.doi.org/10.1111/lam.12002 - ENWEMEKA, C.S., WILLIAMS, D., ENWEMEKA, S.K., HOLLOSI, S. and YENS, D., 2009. Blue 470-nm light kills Methicillin-resistant Staphylococcus aureus (MRSA) in vitro. Photomedicine and Laser Surgery, vol. 27, no. 2, pp. 221-226. http://dx.doi.org/10.1089/pho.2008.2413 PMid:19196103.
» http://dx.doi.org/10.1089/pho.2008.2413 - ENWEMEKA, C.S., WILLIAMS, D., HOLLOSI, S., YENS, D. and ENWEMEKA, S.K., 2008. Visible 405 nm SLD light photo-destroys Methicilin-resistant Staphylococcus aureus (MRSA) in vitro. Lasers in Surgery and Medicine, vol. 40, no. 10, pp. 734-737. http://dx.doi.org/10.1002/lsm.20724 PMid:19065556.
» http://dx.doi.org/10.1002/lsm.20724 - FARIVAR, S., MALEKSHAHABI, T. and SHIARI, R., 2014. Biological effects of low level laser therapy. Journal of Lasers in Medical Sciences, vol. 5, no. 2, pp. 58-62. PMid:25653800.
- FERRER-ESPADA, R., WANG, Y., GOH, X.S. and DAI, T., 2019. Antimicrobial blue light inactivation of microbial isolates in biofilms. Lasers in Surgery and Medicine, In press. http://dx.doi.org/10.1002/lsm.23159 PMid:31536154.
» http://dx.doi.org/10.1002/lsm.23159 - FEUERSTEIN, O., 2012. Light therapy: complementary antibacterial treatment of oral biofilm. Advances in Dental Research, vol. 24, no. 2, pp. 103-107. http://dx.doi.org/10.1177/0022034512449469 PMid:22899690.
» http://dx.doi.org/10.1177/0022034512449469 - FREITAS, C.H., MENDES, J.F., VILLARREAL, P.V., SANTOS, P.R., GONÇALVES, C.L., GONZALES, H.L. and NASCENTE, P.S., 2018. Identification and antimicrobial suceptibility profile of bacteria causing bovine mastitis from dairy farms in Pelotas, Rio Grande do Sul. Brazilian Journal of Biology = Revista Brasileira de Biologia, vol. 78, no. 4, pp. 661-666. http://dx.doi.org/10.1590/1519-6984.170727 PMid:29319754.
» http://dx.doi.org/10.1590/1519-6984.170727 - GUFFEY, J.S. and WILBORN, J., 2006. In vitro bactericidal effects of 405-nm and 470-nm blue light. Photomedicine and Laser Surgery, vol. 24, no. 6, pp. 684-688. http://dx.doi.org/10.1089/pho.2006.24.684 PMid:17199466.
» http://dx.doi.org/10.1089/pho.2006.24.684 - GUFFEY, J.S., PAYNE, W., JONES, T. and MARTIN, K., 2013. Evidence of resistance development by Staphylococcus aureus to an in vitro, multiple stage application of 405 nm light from a supraluminous diode array. Photomedicine and Laser Surgery, vol. 31, no. 4, pp. 179-182. http://dx.doi.org/10.1089/pho.2012.3450 PMid:23484587.
» http://dx.doi.org/10.1089/pho.2012.3450 - HADIS, M.A., ZAINAL, S.A., HOLDER, M.S., CARROLL, J.D., COOPER, P.R., MILWARD, M.R. and PALIN, W.M., 2016. The dark art of light measurement: accurate radiometry for low-level light therapy. Lasers in Medical Science, vol. 31, no. 4, pp. 789-809. http://dx.doi.org/10.1007/s10103-016-1914-y PMid:26964800.
» http://dx.doi.org/10.1007/s10103-016-1914-y - HUANG, Y.Y., SHARMA, S.K., CARROLL, J. and HAMBLIN, M.R., 2011. Biphasic dose response in low level light therapy - an update. Dose-Response, vol. 9, no. 4, pp. 602-618. http://dx.doi.org/10.2203/dose-response.11-009.Hamblin PMid:22461763.
» http://dx.doi.org/10.2203/dose-response.11-009.Hamblin - KIM, S.W., KIM, J.S., LIM, W.B., JEON, S.M., KIM, O.S., KOH, J.T., KIM, C.S., CHOI, H.R. and KIM, O.J., 2013. In vitro bactericidal effects of 625, 525, and 425 nm wavelength (red, green, and blue) light-emitting diode irradiation. Photomedicine and Laser Surgery, vol. 31, no. 11, pp. 554-562. http://dx.doi.org/10.1089/pho.2012.3343 PMid:24138193.
» http://dx.doi.org/10.1089/pho.2012.3343 - KUSHIBIKI, T., HIRASAWA, T., OKAWA, S. and ISHIHARA, M., 2013. Blue laser irradiation generates intracellular reactive oxygen species in various types of cells. Photomedicine and Laser Surgery, vol. 31, no. 3, pp. 95-104. http://dx.doi.org/10.1089/pho.2012.3361 PMid:23390956.
» http://dx.doi.org/10.1089/pho.2012.3361 - LIPOVSKY, A., NITZAN, Y., GEDANKEN, A. and LUBART, R., 2010. Visible light-induced killing of bacteria as a function of wavelength: implication for wound healing. Lasers in Surgery and Medicine, vol. 42, no. 6, pp. 467-472. http://dx.doi.org/10.1002/lsm.20948 PMid:20662022.
» http://dx.doi.org/10.1002/lsm.20948 - MASSON-MEYERS, D.S., BUMAH, V.V., BIENER, G., RAICU, V. and ENWEMEKA, C.S., 2015. The relative antimicrobial effect of blue 405 nm LED and blue 405 nm laser on methicillin-resistant Staphylococcus aureus in vitro. Lasers in Medical Science, vol. 30, no. 9, pp. 2265-2271. http://dx.doi.org/10.1007/s10103-015-1799-1 PMid:26362142.
» http://dx.doi.org/10.1007/s10103-015-1799-1 - MEURER, L., PAYNE, W. and GUFFEY, J.S., 2019. Visible light as an inhibitor of Camplyobacter jejuni. International Journal of Antimicrobial Agents, vol. 55, no. 1, pp. 105818. PMid:31593764.
- OPLÄNDER, C., HIDDING, S., WERNERS, F.B., BORN, M., PALLUA, N. and SUSCHEK, C.V., 2011. Effects of blue light irradiation on human dermal fibroblasts. Journal of Photochemistry and Photobiology. B, Biology, vol. 103, no. 2, pp. 118-125. http://dx.doi.org/10.1016/j.jphotobiol.2011.02.018 PMid:21421326.
» http://dx.doi.org/10.1016/j.jphotobiol.2011.02.018 - PILEGGI, G., WATAHA, J.C., GIRARD, M., GRAD, I., SCHRENZEL, J., LANGE, N. and BOUILLAGUET, S., 2013. Blue light-mediated inactivation of Enterococcus faecalis in vitro. Photodiagnosis and Photodynamic Therapy, vol. 10, no. 2, pp. 134-140. http://dx.doi.org/10.1016/j.pdpdt.2012.11.002 PMid:23769279.
» http://dx.doi.org/10.1016/j.pdpdt.2012.11.002 - ROSA, L.P., SILVA, F.C., VIANA, M.S. and MEIRA, G.A., 2016. In vitro effectiveness of 455-nm blue LED to reduce the load of Staphylococcus aureus and Candida albicans biofilms in compact bone tissue. Lasers in Medical Science, vol. 31, no. 1, pp. 27-32. http://dx.doi.org/10.1007/s10103-015-1826-2 PMid:26498601.
» http://dx.doi.org/10.1007/s10103-015-1826-2 - RUPEL, K., ZUPIN, L., OTTAVIANI, G., BERTANI, I., MARTINELLI, V., PORRELLI, D., VODRET, S., VUERICH, R., PASSOS DA SILVA, D., BUSSANI, R., CROVELLA, S., PARSEK, M., VENTURI, V., DI LENARDA, R., BIASOTTO, M. and ZACCHIGNA, S., 2019. Blue laser light inhibits biofilm formation in vitro and in vivo by inducing oxidative stress. Biofilms and Microbiomes, vol. 5, no. 29, pp. 1-11. http://dx.doi.org/10.1038/s41522-019-0102-9 PMid:31602310.
» http://dx.doi.org/10.1038/s41522-019-0102-9 - SCHMID, J., HOENES, K., VATTER, P. and HESSLING, M., 2019. Antimicrobial effect of visible light-photoinactivation of Legionella rubrilucens by irradiation at 450, 470, and 620 nm. Antibiotics (Basel, Switzerland), vol. 8, no. 187, pp. pii:E187. http://dx.doi.org/10.3390/antibiotics8040187 PMid:31618994.
» http://dx.doi.org/10.3390/antibiotics8040187 - SOUSA, N.T.A., SANTOS, M.F., GOMES, R.C., BRANDINO, H.E., MARTINEZ, R. and GUIRRO, R.R.J., 2015. Blue laser inhibits bacterial growth of Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. Photomedicine and Laser Surgery, vol. 33, no. 5, pp. 278-282. http://dx.doi.org/10.1089/pho.2014.3854 PMid:25954830.
» http://dx.doi.org/10.1089/pho.2014.3854 - WANG, Y., WANG, Y., WANG, Y., MURRAY, C.K., HAMBLIN, M.R., HOOPER, D.C. and DAI, T., 2017. Antimicrobial blue light inactivation of pathogenic microbes: state of the art. Drug Resistance Updates, vol. 33, no. 35, pp. 1-56. http://dx.doi.org/10.1016/j.drup.2017.10.002 PMid:29145971.
» http://dx.doi.org/10.1016/j.drup.2017.10.002 - ZHANG, Y., ZHU, Y., GUPTA, A., HUANG, Y., MURRAY, C.K., VRAHAS, M.S., SHERWOOD, M.E., BAER, D.G., HAMBLIN, M.R. and DAI, T., 2014. Antimicrobial blue light therapy for multidrug-resistant Acinetobacter baumannii infection in a mouse burn model: implications for prophylaxis and treatment of combat-related wound infections. The Journal of Infectious Diseases, vol. 209, no. 12, pp. 1963-1971. http://dx.doi.org/10.1093/infdis/jit842 PMid:24381206.
» http://dx.doi.org/10.1093/infdis/jit842
Publication Dates
-
Publication in this collection
20 May 2020 -
Date of issue
Mar-May 2021
History
-
Received
23 July 2019 -
Accepted
09 Nov 2019