ABSTRACT
Background
The effectiveness of antiviral therapy with pegylated interferon and ribavirin for chronic hepatitis C is far from ideal and presents several adverse events. Among such events, there is the depressive episode that can even lead to treatment discontinuity
Objective
Analyze the incidence of depressive episodes in patients with chronic hepatitis C treated with pegylated interferon (IFN-PEG) and ribavirin, as well as the possible factors associated with its occurrence and its impact on patients' sustained virological response.
Methods
People with chronic hepatitis C undergoing antiviral therapy were interviewed at the baseline, at the 4th, 12th, 24th and 48th treatment weeks and 4 weeks after the end of it, using the HADS scale for tracking the depressive episode. Patients with HADS ≥9 were subjected to Beck Depression Inventory (BDI-II) to grade the episode. Clinical, sociodemographic, laboratorial and histological variables were obtained to identify factors related to the onset of depression. The sustained virological response rate (negative HCV-RNA 6 months after end of therapy) was compared among patients with and without depressive symptoms.
Results
The study comprised 32 patients, most men (59%) with mean age of 54±11.13 years old. Genotype non-1 was prevalent (56%) and 81% of the patients were non-cirrhotic. The depressive episode was diagnosed in 25% of the patients and the peak incidence was found in the 12th treatment week. The depressive episode was moderate in 87% of the patients and only one patient abandoned the treatment. None of the analyzed factors was associated with depressive episode onset. A trend was observed in female patients ( P=0.08). The sustained virological response rate was of 75% and 67% in patients with and without depressive episode, respectively (P =0.66).
Conclusion
The incidence of depressive episodes in patients with chronic hepatitis C undergoing antiviral therapy was of 25% and the 12th treatment week was the most critical one. The presence of depressive episode did not affect the sustained virological response rate.
HEADINGS
Depression; Chronic hepatitis C; Interferon-alpha
RESUMO
Contexto
A terapia antiviral para a hepatite C crônica com interferon peguilado e ribavirina tem eficácia longe do ideal e é repleta de eventos adversos. Entre estes, destaca-se o transtorno depressivo que pode inclusive levar a interrupção do tratamento.
Objetivos
Em pacientes com hepatite C crônica tratados com interferon peguilado (IFN-PEG) e ribavirina, verificar a incidência de episódio depressivo, os possíveis fatores associados ao seu surgimento e o impacto deste sobre a resposta virológica sustentada.
Métodos
Portadores de hepatite C crônica submetidos à terapia antiviral foram entrevistados no Baseline, nas semanas 4, 12, 24, 48 de tratamento e quatro semanas após o término do mesmo utilizando a escala HADS para rastreamento do episódio depressivo e naqueles com HADS ≥9 o Inventário de Depressão de Beck (BDI-II) para graduação do episódio. Variáveis clínicas, sociodemográficas, laboratoriais e histológicas foram obtidas com o objetivo de identificar os fatores relacionados ao surgimento da depressão. A taxa de resposta virológica sustentada (HCV-RNA negativo seis meses após a interrupção da terapia) foi comparada entre os pacientes com e sem sintomas depressivos.
Resultados
Foram incluídos 32 pacientes, a maioria do sexo masculino (59%) e com média de idade de 54±11,13 anos. Prevaleceu o genótipo não 1 (56%) e 81% dos pacientes foram não cirróticos. Episódio depressivo foi diagnosticado em 25% dos pacientes sendo o pico de incidência observado na semana 12 de tratamento. O episódio depressivo foi moderado em 87% dos pacientes e motivou a interrupção em somente 1 deles. Nenhum dos fatores analisados foi associado ao surgimento de episódio depressivo observando-se uma tendência com relação ao sexo feminino ( P =0,08). A taxa de resposta virológica sustentada foi 75% e 67% nos pacientes com e sem episódio depressivo, respectivamente ( P =0,66).
Conclusão
A incidência de episódio depressivo em pacientes com hepatite C crônica submetidos à terapia antiviral foi de 25% e a semana 12 é a mais crítica. A presença de episódio depressivo não interferiu na taxa de resposta virológica sustentada.
DESCRITORES
Depressão; Hepatite C crônica; Interferon-alfa
INTRODUCTION
It is estimated that 170 million people are chronically infected by Hepatitis C Virus (HCV) worldwide and this virus is the leading cause of liver cirrhosis, hepatocellular carcinoma and for the indication for liver transplantation16SBH. Relatório do Grupo de Estudo da Sociedade Brasileira de Hepatologia. Epidemiologia da infecção pelo vírus da hepatite C no Brasil. GED. 1999;18:53-8.. According to the Brazilian Hepatology Society the prevalence of anti-HCV antibody in 1999 was 1.23%16SBH. Relatório do Grupo de Estudo da Sociedade Brasileira de Hepatologia. Epidemiologia da infecção pelo vírus da hepatite C no Brasil. GED. 1999;18:53-8.. Intravenous drug use is nowadays the main contamination route, after the significant reduction in the number of cases related to blood transfusion and blood products14Modabbernia A, Poustchi H, Malekzadeh R. Neuropsychiatric and psychosocial issues of patients with hepatitis C infection: a selective literature review. Hepat Mon. 2013;13:e8340. It is estimated that 15% to 20% of patients with chronic hepatitis C develop liver cirrhosis 20 to 30 years after the infection onset16SBH. Relatório do Grupo de Estudo da Sociedade Brasileira de Hepatologia. Epidemiologia da infecção pelo vírus da hepatite C no Brasil. GED. 1999;18:53-8..
Antiviral therapy is recommended for patients with evidence of progressive disease. Currently, in Brazil, the therapy is based on the use of pegylated interferon (IFN) alpha 2a or 2b and ribavirin associated or not with the protease inhibitors for 24 to 48 weeks depending on the genotype and fibrosis stage. The dual therapy is capable of promoting sustained HCV elimination in 40% to 50% of the patients infected by genotype 1 and in 70% to 80% of those affected by genotypes 2 and 32Asselah T, Marcellin P. New direct-acting antivirals combination for the treatment of chronic hepatitis C. Liver Int. 2011;31:68-77. 6Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-82. 10Manns MP, Mc Hutchison JG,Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-65. . In addition to the inadequate efficacy, the current therapy is associated with the emergence of multiple adverse events such as thyroid dysfunction, cytopenias, flu-like symptoms and neuropsychiatric disorders, especially depressive episodes6Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-82..
The mechanism by which the INF triggers the depressive episode is uncertain, but it possibly causes increase in the concentration of cortisol and interleukin-6 (IL-6) and changes tryptophan and serotonin levels, which are key neurotransmitters in the genesis of mood changes18Wichers MC, Koek GH, Robaeys G, et al. IDO and interferon- alpha induced depressive symptoms a shift in hypothesis from tryptophan depletion to neurotoxicity.. Mol Psychiatry 2005;10:538-44. 19Wichers MC, Koek GH, Robaeys G, et al. Interferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol.. J Psychosom Res 2007;62: 207-14.. Moreover, it is possible that HCV makes the neurochemical systems vulnerable to the action of INF and interleukins modulated by it18Wichers MC, Koek GH, Robaeys G, et al. IDO and interferon- alpha induced depressive symptoms a shift in hypothesis from tryptophan depletion to neurotoxicity.. Mol Psychiatry 2005;10:538-44. 19Wichers MC, Koek GH, Robaeys G, et al. Interferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol.. J Psychosom Res 2007;62: 207-14..
The depressive episode onset during treatment may compromise adherence, quality of life and reduce the chances of eradicating the virus19Wichers MC, Koek GH, Robaeys G, et al. Interferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol.. J Psychosom Res 2007;62: 207-14.. According to the literature, 15% to 44% of patients treated with pegylated interferon and ribavirin develop neuropsychiatric disorders5Fontana RJ, Kronfol Z, Lindsay KL, et al. Changes in Mood States and Biomarkers During Peginterferon and Ribavirin Treatment of Chronic Hepatitis C. AMJ Gastroenterol. 2008;103:2766-75. 9Lotrich FE, Rabinovitz M, Gironda P, et al. Depression following pegylated interferon alpha: characteristics and vulnerability.. J Psychosom Res 2007;63:131-35. 13Medeiros LPJ, Kayo M, Medeiros RBV, et al. Interferon-induced depression in patients with hepatitis C: an epidemiologic study. Rev Assoc Med Bras. 2014;60.. According to Schaefer et al., Interferon-induced depression is the most important adverse event and it may lead to early therapy discontinuity17Schaefer M, Capuron L, Friebe A, et al. Hepatitis C infection, antiviral treatment and mental health: A European expert consensus statement. J Hepatol. 2012;57:1379-90..
Therefore, the current study aims to prospectively evaluate the incidence of depressive episodes and the factors associated with their occurrence in patients with Chronic Hepatitis C undergoing antiviral therapy with pegylated interferon and ribavirin in a hepatology clinic of a public university hospital, as well as to investigate the relation between the presence of depressive episodes and the sustained virological response rate.
METHODS
A prospective cohort study was conducted and it consecutively included patients with chronic hepatitis C undergoing antiviral therapy with pegylated interferon alpha 2a or 2b and ribavirin, regular folow up in the Gastroenterology Department Hepatology Unit of the HU/CAS - Federal University of Juiz de Fora, from June 2012 to June 2014. Patients with depressive symptoms detected at the baseline, patients with chronic kidney disease, hypothyroidism and co-infected with HIV and HBV were excluded from the study.
Patients underwent clinical evaluation and were interviewed by the same psychologist immediately prior to treatment initiation (baseline) in order to obtain the clinical, laboratorial, histological and sociodemographic variables (family history or personal history of depression, history of treatment with IFN, age, gender, color, educational level, profession, employment status, mode of infection acquisition, genotype and HCV viral load and fibrosis stage, for histology according to Metavir classification). The sustained virological response (SVR) was considered the negative RNA-HCV results 24 weeks after the end of the treatment.
Patients were subjected to Hospital Anxiety and Depression Scale (HADS) - in its translated and validated version for the Brazilian population - to identify depressive episodes and - in the presence of it - the Beck Depression Inventory was applied in order to the grade the episode (BDI -II)4Cunha JA. Manual da versão em português das Escalas Beck. 2001. São Paulo: Casa do Psicólogo. 7Gorenstein C, Andrade L. Validation of a Portuguese version of the Beck depression inventory and the state-trait anxiety inventory in Brazilian subjects. Braz J Med Biol Res. 1996;29:453-57. 11Marcolino JAM, Mathias LAST, Filho LP, et al. Escala Hospitalar de Ansiedade e Depressão: Estudo da Validade de Critério e da Confiabilidade com Pacientes no Pré-Operatório. Rev Bras Anestesiol. 2007;57:52-62. 20Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand.1983;67:361-70.. HADS was applied at the baseline, in the 4th, 12th, 24th and 48th treatment weeks and 4 weeks after the end of the treatment. HADS consists of 14 items, seven of them for identifying anxiety (HADS-A) and seven for screening depressive symptoms (HADS-D) and it just takes into account the psychic symptoms11Marcolino JAM, Mathias LAST, Filho LP, et al. Escala Hospitalar de Ansiedade e Depressão: Estudo da Validade de Critério e da Confiabilidade com Pacientes no Pré-Operatório. Rev Bras Anestesiol. 2007;57:52-62. 20Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand.1983;67:361-70.. Patients with HADS-D ≥912Martíns-Santos R, Díez-Quevedo C, Castellví P, et al. De novo depression and anxiety disorders and influence on adherence during peginterferon-alpha-2a and ribavirin treatment in patients with hepatitis C. Aliment Pharmacol Ther. 2008;27: 257-65. 20Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand.1983;67:361-70. were considered as having depressive episode. Patients with HADS-D ≥9 underwent self-assessment using Beck Inventory (BDI-II). The BDI-II consists of 21 items and it was used in order to grade the depressive episode levels as minimum (0-13), mild (14-19), moderate (20-28) and severe (29-63)4Cunha JA. Manual da versão em português das Escalas Beck. 2001. São Paulo: Casa do Psicólogo. 7Gorenstein C, Andrade L. Validation of a Portuguese version of the Beck depression inventory and the state-trait anxiety inventory in Brazilian subjects. Braz J Med Biol Res. 1996;29:453-57.. The analysis just included patients with at least 12 weeks of treatment. The grading scales were applied to patients with early therapy termination four weeks after therapy interruption.
The study was approved by the Ethics Research Committee of the University Hospital at the Federal University of Fora/MG. All participants were included in the study after reading and signing the Informed Consent Form.
Statistical analysis
The statistical analysis was performed using SPSS software version 20.0 for Windows (SPSS Chicago IL USA). Categorical variables were expressed as absolute (n) and relative (%) frequency. The chi-square test was used and, when it was appropriate, the Fisher's test was used to analyze the categorical variables. Numerical variables were expressed as mean or median and standard deviation. The significance level of 0.05 (α=5%) was adopted. Descriptive levels equal to or less than this value were considered significant. Furthermore, the survival analysis approach was used. The log-rank test, the cumulative risk curves by Kaplan Meier as well as the proportional hazards model by Cox were used to compare the curves.
RESULTS
Thirty-two (32) out of the 40 initially selected patients were included in the study. Eight patients were excluded from it because they did not attend the scheduled assessments. The mean age was 54±11.7 years old and 59% of the patients were male, 69% of them were married and 62% of them had active working life. Blood transfusion and/or blood products were the most common HCV contamination routes (34.4%), whereas 22% of the patients were infected through injected and/or inhaled drugs. Fifty-three percent (53%) of the studied population showed at least one comorbidity mainly represented by diabetes mellitus type II and arterial Hypertension. Most patients (56%) were infected by genotypes 2 and 3 and 81% of them showed no evidence of liver cirrhosis. The treatment lasted 12, 24 and 48 weeks in 16%, 50% and 34% of patients, respectively, with mean duration of 30 weeks. Early therapy discontinuity occurred in five (16%) patients due to adverse events. One out of these five patients discontinued the therapy due to depression. The sustained virological response rate was of 19% in patients with genotype 1 and 50% in patients with genotype non-1 (Table 1).
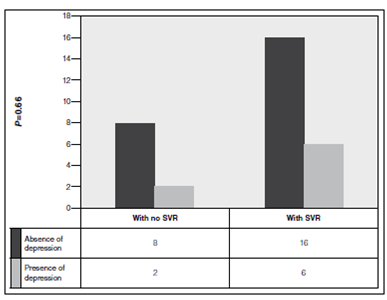
The presence of depressive episode occurred in 25% of the patients. The peak incidence was observed in the 12th therapy week. According to the BDI, 87% of the episodes were considered moderate. Among depressed patients, 50% were female and 50% were married. Most of them was employed (75%), and showed no liver cirrhosis (75%). There was a tendency of association between depressive episode and female gender (P =0.08), this result was highlighted since regarding the general population, depression is more prevalent in women (Figure 1). Any of the analyzed variables was associated with the onset of depressive episodes (Table 2). Only one patient remained depressed 4 weeks after the end of the treatment. It is noteworthy that five patients diagnosed with depressive episode, according to HADS, received antidepressants after week 12, as recommended by the assisting physicians who were unaware of the results from the applied scales. The sustained virological response rate was of 75% in patients with depressive episode and 67% in those without depressive episodes ( P =0.66) (Figure 2 and 3).
Sustained virological response (SVR) rate according to the presence of depressive episode (n=32)
DISCUSSION
Results from the current study show that the incidence of depressive episodes in Brazilian patients with chronic hepatitis C treated with PEG IFN alpha and ribavirin, which showed no depression at the time they were included in the treatment is high; a fact that is consistent with previously published studies. The present study included just patients without depressive symptoms (HADS-D <9) at the baseline in order to reliably associate depressive episodes with antiviral therapy.
The emergence of depressive episode related to the use of IFN, although its mechanism is still not fully elucidated, is common among in-treatment patients with chronic hepatitis C. A study by Lotrich et al. comprised 23 patients and used the Structured Clinical Interview for DSM IV (SCID) and self-report questionnaires to evaluate them. The authors identified depressive episode in 39% of the euthymic patients (with no history of depression in the past six months) after 3 months of therapy with pegylated IFN alpha9Lotrich FE, Rabinovitz M, Gironda P, et al. Depression following pegylated interferon alpha: characteristics and vulnerability.. J Psychosom Res 2007;63:131-35.. Fontana et al. related the presence of depressive disorder among 201 individuals with chronic hepatitis C, according to the Beck Depression Inventory and the Composite International Diagnostic Interview, in 23% of the patients in the 24th week and in 42% of them in the 48th week of antiviral therapy with PEG IFN and ribavirin5Fontana RJ, Kronfol Z, Lindsay KL, et al. Changes in Mood States and Biomarkers During Peginterferon and Ribavirin Treatment of Chronic Hepatitis C. AMJ Gastroenterol. 2008;103:2766-75.. In Brazil, a prospective study performed by Medeiros et al. included 50 patients with chronic hepatitis C treated with PEG IFN and ribavirin. These patients were evaluated using the Beck Depression Inventory (BDI) and the Population Screening Depression Scale (CES-D) and 46% of them showed depression13Medeiros LPJ, Kayo M, Medeiros RBV, et al. Interferon-induced depression in patients with hepatitis C: an epidemiologic study. Rev Assoc Med Bras. 2014;60.. The present study found that 25% of the patients were diagnosed with depressive disorder at week 12, according to the HADS scale.
Since the depressive episode onset may jeopardize treatment continuity, it is important to determine the moment when its presence is more frequent. According to the literature, this moment may vary, but it preferably falls in the first half of the treatment. The current study found that the peak incidence occurred at week 12. Beratis et al. identified the presence of depression in 31% of the patients in the fourth treatment week whereas Martins-Santos et al. reported that over 90% of depressed individuals developed depression during treatment with PEG-IFN within the first 12 weeks3Beratis S, Katrivanou A, Georgiou S, et al. Major depression and risk of depressive symptomatology associated with short-term and low-dose interferon-alpha treatment. J Psychosom Res. 2005;58:15-8. 12Martíns-Santos R, Díez-Quevedo C, Castellví P, et al. De novo depression and anxiety disorders and influence on adherence during peginterferon-alpha-2a and ribavirin treatment in patients with hepatitis C. Aliment Pharmacol Ther. 2008;27: 257-65.. Hauser et al. used the BDI and found that the frequency of depression was higher between 6 and 22 weeks of treatment8Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;27:942-7.. A recent study published by Medeiros et al. found that the emergence of depression peaked in the 24th treatment week13Medeiros LPJ, Kayo M, Medeiros RBV, et al. Interferon-induced depression in patients with hepatitis C: an epidemiologic study. Rev Assoc Med Bras. 2014;60.. Therefore, it is recommended that the assistant medical team be aware to the development of depressive episodes, especially in the first half of the antiviral therapy.
It was not possible to identify the predictive emergence of depressive symptoms in the studied sample. A study by Araújo, who included patients that were not undergoing antiviral therapy, found that age was the only variable associated with the emergence of depressive disorder, which was more common in patients under the age of 60(1). Pavlovic et al. conducted a study with 74 patients with chronic hepatitis C undergoing antiviral therapy with pegylated interferon for 24 weeks and identified depressive symptoms in 34% of them. However, it was not possible to identify factors associated with the onset of these symptoms15Pavlovic Z, Delic D, Nadja P, et al. Depressive symptoms in patients with hepatitis C treated with pegylated interferon alpha therapy:a 24-week prospective study. Psychiatria Danubina. 2011:23:370-377..
As for the sustained virological response, it is possible that the depressive episode onset affects the chance of permanently eradicating HCV. One of the justifications for this fact is that the depressive episode possibly compromises the adherence to therapy, which is critical for achieving SVR. The adherence rate was not systematically checked in the current study, but it was always valued and encouraged during the consultations, throughout the treatment. The SVR rate was of 75% in patients with depressive episode and 67% in those without depression (P =0.66). Therefore, the development of depressive episodes did not influence the sustained virological response rate. It is noteworthy that 62% of the patients with depressive episode, who were diagnosed according to HADS, received antidepressant after the 12th therapy week, as recommended by the assistant medical team who did not know the results from HADS scale. Such fact may have contributed to the maintenance of SVR rates in patients who developed depression, but it highlights the importance of applying specific questionnaires for diagnosing depressive episodes, since a significant number of patients did not receive specific treatment.
Although the current study prospectively showed that the incidence of depressive episodes in treated patients is significant, it should be noticed that further studies with larger samples are needed in order to confirm the results. The small number of treated patients and the predominance of genotypes 2 and 3 in this group occurred because the study period met the prospect of releasing new drugs (Boceprevir and Telaprevir) for patients with genotype 1 who, therefore, were awaiting the triple therapy to be put available.
In conclusion, it is recommended that patients undergoing antiviral therapy with pegylated interferon should be systematically evaluated in search for depressive episode, especially in the first 12 weeks of therapy, since the depressive episode affects a significant number of treated patients. The presence of depressive disorder has no negative impact on the sustained virological response rate.
REFERENCES
- Araújo RF. Prevalência do Transtorno Depressivo Maior em pacientes com Hepatite C Crônica e características psicométricas de instrumentos diagnósticos para o rastreamento de quadros depressivos [dissertação]. Belo Horizonte: UFMG, 2012.
- Asselah T, Marcellin P. New direct-acting antivirals combination for the treatment of chronic hepatitis C. Liver Int. 2011;31:68-77.
- Beratis S, Katrivanou A, Georgiou S, et al. Major depression and risk of depressive symptomatology associated with short-term and low-dose interferon-alpha treatment. J Psychosom Res. 2005;58:15-8.
- Cunha JA. Manual da versão em português das Escalas Beck. 2001. São Paulo: Casa do Psicólogo.
- Fontana RJ, Kronfol Z, Lindsay KL, et al. Changes in Mood States and Biomarkers During Peginterferon and Ribavirin Treatment of Chronic Hepatitis C. AMJ Gastroenterol. 2008;103:2766-75.
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-82.
- Gorenstein C, Andrade L. Validation of a Portuguese version of the Beck depression inventory and the state-trait anxiety inventory in Brazilian subjects. Braz J Med Biol Res. 1996;29:453-57.
- Hauser P, Khosla J, Aurora H, et al. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Mol Psychiatry. 2002;27:942-7.
- Lotrich FE, Rabinovitz M, Gironda P, et al. Depression following pegylated interferon alpha: characteristics and vulnerability.. J Psychosom Res 2007;63:131-35.
- Manns MP, Mc Hutchison JG,Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-65.
- Marcolino JAM, Mathias LAST, Filho LP, et al. Escala Hospitalar de Ansiedade e Depressão: Estudo da Validade de Critério e da Confiabilidade com Pacientes no Pré-Operatório. Rev Bras Anestesiol. 2007;57:52-62.
- Martíns-Santos R, Díez-Quevedo C, Castellví P, et al. De novo depression and anxiety disorders and influence on adherence during peginterferon-alpha-2a and ribavirin treatment in patients with hepatitis C. Aliment Pharmacol Ther. 2008;27: 257-65.
- Medeiros LPJ, Kayo M, Medeiros RBV, et al. Interferon-induced depression in patients with hepatitis C: an epidemiologic study. Rev Assoc Med Bras. 2014;60.
- Modabbernia A, Poustchi H, Malekzadeh R. Neuropsychiatric and psychosocial issues of patients with hepatitis C infection: a selective literature review. Hepat Mon. 2013;13:e8340
- Pavlovic Z, Delic D, Nadja P, et al. Depressive symptoms in patients with hepatitis C treated with pegylated interferon alpha therapy:a 24-week prospective study. Psychiatria Danubina. 2011:23:370-377.
- SBH. Relatório do Grupo de Estudo da Sociedade Brasileira de Hepatologia. Epidemiologia da infecção pelo vírus da hepatite C no Brasil. GED. 1999;18:53-8.
- Schaefer M, Capuron L, Friebe A, et al. Hepatitis C infection, antiviral treatment and mental health: A European expert consensus statement. J Hepatol. 2012;57:1379-90.
- Wichers MC, Koek GH, Robaeys G, et al. IDO and interferon- alpha induced depressive symptoms a shift in hypothesis from tryptophan depletion to neurotoxicity.. Mol Psychiatry 2005;10:538-44.
- Wichers MC, Koek GH, Robaeys G, et al. Interferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol.. J Psychosom Res 2007;62: 207-14.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand.1983;67:361-70.
-
3
Disclosure of funding: no funding received
Publication Dates
-
Publication in this collection
Jan-Mar 2016
History
-
Received
09 May 2015 -
Accepted
01 Oct 2015