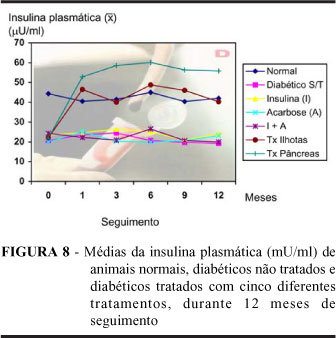
PURPOSE: The long-term effects of five different treatments of diabetes were evaluated in alloxan-induced diabetic rats. METHODS: Seven experimental groups, with 50 rats each (GN normal control; GD untreated diabetic control; GI, GA, GIA treated groups with insulin, acarbose, and insulin plus acarbose, respectively; GTIL, GTPD treated groups with islet of Langerhans and pancreas transplantation) were studied. Clinical (body weight, water intake, food intake and urine output) and laboratory (blood and urinary glucose, and plasma insulin) parameters were analyzed at the beginning of the study, and after 1, 3, 6, 9 and 12 months of follow-up. RESULTS: Mortality was observed in all groups, except GN, during 12 months (GD= 50%; GI= 20%; GA= 26%; GIA= 18%; GTIL= 4%; GTPD= 20%). Rats from the GD, GI, and GIA groups died due to metabolic or hydrossaline disbalance, and/or pneumonia, diarrhoea, and cachexy. All deaths observed in GTIL and GTPD groups were in decorrence of technical failure at the immediate postoperative, until 72h. Animals from the GI, GA and GIA had significative improving of the clinical and laboratory parameters (p < 0,05) observed in diabetic rats, being the efficacy of theses treatments equal. However, rats from the GTIL and GTPD groups had better control of these parameters than GI, GA, and GIA groups. Transplanted rats had complete restoration, at the normal levels, of all analyzed variables (p<0,01). CONCLUSIONS: Conventional treatments with insulin, acarbose, and insulin plus acarbose improved the severe diabetic state of the alloxan-diabetic rats, but pancreas and islet transplantation have a better performance for treatment of diabetes.
Diabetes; Rats; Alloxan; Insulin; Acarbose; Pancreas Transplantation; Islet of Langerhans Transplantation