Abstract
Purpose:
To investigate the specific molecular mechanisms and effects of curcumin derivative J147 on diabetic peripheral neuropathy (DPN).
Methods:
We constructed streptozotocin (STZ)-induced DPN rat models to detected mechanical withdrawal threshold (MWT) in vivo using Von Frey filaments. In vitro, we measured cell viability and apoptosis, adenosine 5‘-monophosphate-activated protein kinase (AMPK) and transient receptor potential A1 (TRPA1) expression using MTT, flow cytometry, qRT-PCR and western blot. Then, TRPA1 expression level and calcium reaction level were assessed in agonist AICAR treated RSC96cells.
Results:
The results showed that J147reduced MWT in vivo, increased the mRNA and protein level of AMPK, reduced TRPA1 expression and calcium reaction level in AITCR treated RSC96 cells, and had no obvious effect on cell viability and apoptosis. Besides, AMPK negative regulated TRPA1 expression in RSC96 cells.
Conclusions:
J147 could ameliorate DPN via negative regulation AMPK on TRPA1 in vivo and in vitro. A curcumin derivative J147might be a new therapeutic potential for the treatment of DPN.
Key words:
Curcumin; Peripheral Nervous System Diseases; TRPA1 Cation Channel; Protein Kinases; Rats.
Introduction
Diabetic peripheral neuropathy (DPN) is one of the major disability factors and the most common chronic neurological complications in diabetes11 El Boghdady NA, Badr GA. Evaluation of oxidative stress markers and vascular risk factors in patients with diabetic peripheral neuropathy. Cell Biochem Funct. 2012;30(4):328-34. doi:10.1002/cbf.2808.
https://doi.org/10.1002/cbf.2808...
. The clinical manifestations of this disease are distal symmetric pain with paresthesia, like numbness, ant walking, insect crawling, fever, electric shock, and motor neurological disorders22 Wooten K. Clinical features and electrodiagnosis of diabetic peripheral neuropathy in the dysvascular patient. Phys Med Rehabil Clin N Am. 2009;20(4):657-76. doi: 10.1016/j.pmr.2009.06.011.
https://doi.org/10.1016/j.pmr.2009.06.01...
,33 Piao Y, Liang X. Chinese medicine in diabetic peripheral neuropathy: experimental research on nerve repair and regeneration. Evid Based Complement Alternat Med. 2012;2012:191632. doi: 10.1155/2012/191632.
https://doi.org/10.1155/2012/191632...
.Further, severe DPN patients may develop lower extremity arthropathy and ulcers22 Wooten K. Clinical features and electrodiagnosis of diabetic peripheral neuropathy in the dysvascular patient. Phys Med Rehabil Clin N Am. 2009;20(4):657-76. doi: 10.1016/j.pmr.2009.06.011.
https://doi.org/10.1016/j.pmr.2009.06.01...
. In addition, there will be DPN in 30-50% diabetic patients by 2025 according to the prediction from WHO44 Tesfaye S. Advances in the management of diabetic peripheral neuropathy. Curr Opin Support Palliat Care. 2009 Jun;3(2):136-43. doi: 10.1097/SPC.0b013e32832b7df5.
https://doi.org/10.1097/SPC.0b013e32832b...
. Therefore, in order to ameliorate the patient’s clinical symptoms and control the development of DPN, the comprehensive treatments are used, such as control of blood glucose, oral Duloxetine and Pregabalin, interventional therapy and surgical treatment55 Martin CL, Albers J, Herman WH, Cleary P, Waberski B, Greene DA, Stevens MJ, Feldman EL. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care. 2006;29(2):340. PMID: 16443884.
6 Gutierrez-Alvarez AM, Beltrán-Rodríguez J, Moreno CB. Antiepileptic drugs in treatment of pain caused by diabetic neuropathy. J Pain Symptom Manage. 2007 Aug;34(2):201-8 doi: 10.1016/j.jpainsymman.2006.10.023.
https://doi.org/10.1016/j.jpainsymman.20...
7 De CV, Rajan V, Steenbergen W, He DAV, Buschman HP. Effect and safety of spinal cord stimulation for treatment of chronic pain caused by diabetic neuropathy. J Diabetes Complications. 2009 Jan-Feb;23(1):40-5. doi: 10.1016/j.jdiacomp.2007.08.002.
https://doi.org/10.1016/j.jdiacomp.2007....
-88 Dellon AL. The Dellon approach to neurolysis in the neuropathy patient with chronic nerve compression. Handchir Mikrochir Plast Chir. 2008 Dec;40(6):351-60. doi: 10.1055/s-2008-1039216. PMID:19051159.
https://doi.org/10.1055/s-2008-1039216...
. Regardless, the exact pathogenesis of DPN is not fully understood until now. Some studies have reported that this disease is a combination of multiple factors, including metabolic disorders, vascular injury, neurotrophic factor deficiency, cytokine abnormalities, oxidative stress and immune factors11 El Boghdady NA, Badr GA. Evaluation of oxidative stress markers and vascular risk factors in patients with diabetic peripheral neuropathy. Cell Biochem Funct. 2012;30(4):328-34. doi:10.1002/cbf.2808.
https://doi.org/10.1002/cbf.2808...
,33 Piao Y, Liang X. Chinese medicine in diabetic peripheral neuropathy: experimental research on nerve repair and regeneration. Evid Based Complement Alternat Med. 2012;2012:191632. doi: 10.1155/2012/191632.
https://doi.org/10.1155/2012/191632...
,99 Lu You, Lu Ying-li. Progress in pathogenesis of diabetic peripheral neuropathy. J Shanghai Jiaotong Univ. 2017;37(10):1441. doi: 10.3969/j.issn.1674-8115.2017.10.027.
https://doi.org/10.3969/j.issn.1674-8115...
. However, a study of Guastella and Mick1010 Guastella V, Mick G. Strategies for the diagnosis and treatment of neuropathic pain secondary to diabetic peripheral sensory polyneuropathy. Diabetes Metab. 2009;35(1):12-9. doi: 10.1016/j.diabet.2008.09.003.
https://doi.org/10.1016/j.diabet.2008.09...
has shown that no medication could prevent or reverse the DPN progression, or promote nerve regeneration. Meanwhile, there is also not enough data to support that preventive or potential treatment measures could reduce the incidence of neuropathic pain in diabetic patients1010 Guastella V, Mick G. Strategies for the diagnosis and treatment of neuropathic pain secondary to diabetic peripheral sensory polyneuropathy. Diabetes Metab. 2009;35(1):12-9. doi: 10.1016/j.diabet.2008.09.003.
https://doi.org/10.1016/j.diabet.2008.09...
.
Curcumin is a natural product that has extensive biological activities and cellular mechanisms, while plays an effective role in the treatment of diseases such as Alzheimer’s disease, cancers, diabetes, DPN and inflammation1111 Lakeybeitia J, González Y, Doens D, Stephens DE, Santamaría R, Murillo E, Gutiérrez M, Fernández PL, Rao KS, Larionov OV. Assessment of novel curcumin derivatives as potent inhibitors of inflammation and amyloid-ß aggregation in Alzheimer's disease. J Alzheimers Dis. 2017;60(s1):S59-S68. doi: 10.3233/JAD-170071.
https://doi.org/10.3233/JAD-170071...
12 Baloch Z, Yasmeen N, Li Y, Zhang W, Lu H, Wu X, Xia X, Yang S. Knowledge and awareness of cervical cancer, human papillomavirus (HPV), and HPV vaccine among HPV-infected chinese women. Med Sci Monit. 2017;23:4269-77. PMID: 28867818.
13 Srinivasan M. Effect of curcumin on blood sugar as seen in a diabetic subject. Indian J Med Sci. 1972;26(4):269-70. PMID: 4637293.-1414 Banafshe HR, Hamidi GA, Noureddini M, Mirhashemi SM, Mokhtari R, Shoferpour M. Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol. 2014;723:202-6. doi: 10.1016/j.ejphar.2013.11.033.
https://doi.org/10.1016/j.ejphar.2013.11...
. Cocorocchio et al.1515 Cocorocchio M, Baldwin AJ, Stewart B, Kim L, Harwood AJ, Thompson CRL, Andrews PLR, Williams RSB. Curcumin and derivatives function through protein phosphatase 2A and presenilin orthologues in Dictyostelium discoideum. Dis Model Mech. 2018 Jan 29;11(1). doi: 10.1242/dmm.032375.
https://doi.org/10.1242/dmm.032375...
attests that curcumin can act on cells in an acute manner, reduce cell growth and slow down multicellular development.
In 2011, Chen and Prior et al.1616 Chen Q, Prior M, Dargusch R, Roberts A, Riek R, Eichmann C, Chiruta C, Akaishi T, Abe K, Maher P, Schubert D. A novel neurotrophic drug for cognitive enhancement and Alzheimer's disease. PLoS One. 2011;6(12):e27865. doi: 10.1371/journal.pone.0027865.
https://doi.org/10.1371/journal.pone.002...
discovered curcumin derivative J147 (CAS No.: 1146963-51-0) when screening compounds from plants with the ability to reverse brain aging cells and molecular marker. Several reports suggest that J147 can reverse memory deficits, enhance the production of new brain cells, and retard the progression of Alzheimer’s disease in rats1616 Chen Q, Prior M, Dargusch R, Roberts A, Riek R, Eichmann C, Chiruta C, Akaishi T, Abe K, Maher P, Schubert D. A novel neurotrophic drug for cognitive enhancement and Alzheimer's disease. PLoS One. 2011;6(12):e27865. doi: 10.1371/journal.pone.0027865.
https://doi.org/10.1371/journal.pone.002...
,1717 Goldberg J, Currais A, Prior M, Fischer W, Chiruta C, Ratliff E, Daugherty D, Dargusch R, Finley K, Esparza-Molto PB, Cuezva JM, Maher P, Petrascheck M, Schubert D. The mitochondrial ATP synthase is a shared drug target for aging and dementia. Aging Cell. 2018;17(2). doi: 10.1111/acel.12715.
https://doi.org/10.1111/acel.12715...
.Related the study of molecular mechanisms have found that J147 combines with the proteins mitochondrial proteins via ATP synthase, there by promoting aging cells, mice and fruit flies to look younger1717 Goldberg J, Currais A, Prior M, Fischer W, Chiruta C, Ratliff E, Daugherty D, Dargusch R, Finley K, Esparza-Molto PB, Cuezva JM, Maher P, Petrascheck M, Schubert D. The mitochondrial ATP synthase is a shared drug target for aging and dementia. Aging Cell. 2018;17(2). doi: 10.1111/acel.12715.
https://doi.org/10.1111/acel.12715...
. Besides, Lian et al.1818 Lian L, Xu Y, Zhang J, Yu Y, Zhu N, Guan X, Huang H, Chen R, Chen J, Shi G, Pan J. Antidepressant-like effects of a novel curcumin derivative J147: Involvement of 5-HT1A receptor. Neuropharmacology. 2018;135:506-13. doi: 10.1016/j.neuropharm.2018.04.003.
https://doi.org/10.1016/j.neuropharm.201...
reports that J147 improves the depression-like behavior in mouse models caused by chronic stress, and its mechanism may involve the regulation of cAMP/pCREB/BDNF pathway.
Due to curcumin can relieve diabetic peripheral neuropathic pain1414 Banafshe HR, Hamidi GA, Noureddini M, Mirhashemi SM, Mokhtari R, Shoferpour M. Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol. 2014;723:202-6. doi: 10.1016/j.ejphar.2013.11.033.
https://doi.org/10.1016/j.ejphar.2013.11...
, we aimed to investigate the specific molecular mechanisms and effects of J147 on DPN through the establishment of DPN rat models, cell viability and apoptosis assay in this study.
Methods
DPN rat models
Ethical Guidelines were approved by the Ethical Committee of Shanxi University of Traditional Chinese Medicine.
The 30 adult male SPF rats (weighing 200 ± 20g) were obtained from Institute of Laboratory Animal Science, Chinese Academy of Medical Science (Beijing, China). They were housed in stainless steel squirrel cages with food and water available ad libitum. The breeding room was kept at 20°C, 70% humidity and 12 h of light.SPF rats were weighed after fasting for 12 h, and streptozotocin(STZ) solution (Solarbio, Beijing, China) was injected intraperitoneally at 50 mg/kg weight. After 4 h of injection, the rats resumed their diet and were given water with glucose for 6 h, and then blood sugar was measured after 72 h. Subsequently, DPN rats were randomly divided into 3 groups: one group was fed with 10 μM of J147 (Myland Biological Technology Co., Ltd., Suzhou, China) at 10 mg/kg weight, the other group was fed with 100 μM of J147, while the control group was fed the same amount of normal saline1414 Banafshe HR, Hamidi GA, Noureddini M, Mirhashemi SM, Mokhtari R, Shoferpour M. Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol. 2014;723:202-6. doi: 10.1016/j.ejphar.2013.11.033.
https://doi.org/10.1016/j.ejphar.2013.11...
,1919 Prior M, Dargusch R, Ehren JL, Chiruta C, Schubert D. The neurotrophic compound J147 reverses cognitive impairment in aged Alzheimer's disease mice. Alzheimers Res Ther. 2013;5(3):25. doi: 10.1186/alzrt179.
https://doi.org/10.1186/alzrt179...
. Then, to evaluate the hypersensitivity of mechanical pain after medication, mechanical withdrawal threshold (MWT) of algesia at 1, 2, 3, 4 and 5 days after feeding J147 were measured using Von Frey filaments (DanMic Global, California, USA).
Cell culture
RSC96 cells were purchased from ATCC (Manassas, USA) and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, Thermo Fisher Scientific, Waltham, USA) with10% fetal bovine serum (FBS, Thermo Fisher Scientific, Waltham, USA), dimethylsulfoxide (DMSO, Thermo Fisher Scientific, Waltham, USA) and 150 mM of high-glucose (Thermo Fisher Scientific, Waltham, USA). The cells were divided into 3 groups, and respectively treated with J147 (10 and 100 μM) and PBS at 37°C. In addition, each group of partial cells was divided into two parts for follow-up experiments. One part was added 1 mM AMPK activator AICAR (AICAR+, Sigma-Aldrich, St. Louis, USA), while the control group was not added AICAR (AICAR-).
Cell viability assay
In order to detect the growth of cells, MTT Cell Proliferation and Cytotoxicity Assay Kit (Sangon Biotech, Shanghai, China) was used. Cells were seeded in 96-well plate (2000 cells/well) for 24 h. After adding 5 mg/ml of MTT solution to each well, cells were incubated with 5% CO2at 37°C for 4 h, and were added to 100 μl of formazan solubilization solution. Then, the absorbance was measured at 450 nm (OD450) by microplate reader (DeTie, Nanjing, China).
Apoptosis assay
Cell apoptosis was measured using Annexin V-FITC/PI Apoptosis Assay Kit (Sangon Biotech, Shanghai, China). Cells (2 × 1055 Martin CL, Albers J, Herman WH, Cleary P, Waberski B, Greene DA, Stevens MJ, Feldman EL. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care. 2006;29(2):340. PMID: 16443884. cells/ml) were re-suspended in195 μl of 1 × Binding Buffer. Then, cell suspension were added to 5 μl of Annexin V-FITC and incubated for 10-15 min at the room temperature. Cells were washed in 1 × Binding Buffer twice, re-suspended again and added to 10 μl of PI. Finally, flow cytometry (Thermo Fisher Scientific, Waltham, USA) was used to observe the fluorescence within 4 h, and the percentage of apoptosis was calculated.
Quantitative real-time polymerase chain reaction (qRT-PCR) assay
RSC96 cells were cleaved byTrizol solution (Thermo Fisher Scientific, Waltham, USA) and incubated for 5 min at the room temperature. The RNA was precipitated with chloroform and isopropanol (Thermo Fisher Scientific, Waltham, USA), washed three times in 75% ethanol (Thermo Fisher Scientific, Waltham, USA), and was transcribed into cDNA using All-in-OneTM First Strand cDNA Synthesis Kit (GeneCopoeia, Rockville, USA). Then, 4 μl of cDNA was combined with primers(β-actin as internal reference)to perform PCR under conditions of 95°C for5 min, 94°C for 30 s, 55°C for40 s and 72°C for1 min. The 10μlof product was electrophoresed and photographed using 1% agarose gel (Thermo Fisher Scientific, Waltham, USA). The grayscale was measured by ImageTool (IT) 3.0 (University of Texas Health Science Center, San Antonio, USA), and the relative mRNA expression level was calculated.
Western blot assay
RSC96 cells were lysed using RIPA Lysis Buffer (Beyotime, Shanghai, China). Lysed cell fluid was detected the protein concentration using Enhanced BCA Protein Assay Kit (Beyotime, Shanghai, China). Then, the protein samples were added 2 × SAS-PAGE Sample Loading Buffer (Beyotime, Shanghai, China), heated for 3-5 min at 100°C, and transferred to Polyvinylidene Difluoride (PVDF) membrane for 30 min using Bio-rad Trans-Blot SD (Bio-Rad, Hercules, USA). Subsequently, the protein membrane was washed in Western Wash Buffer (Beyotime, Shanghai, China) for 1 min and added Western Blocking Buffer (Beyotime, Shanghai, China) for 1 h at the room temperature. The primary antibodies of Adenosine 5‘-monophosphate-activated protein kinase (AMPK), transient receptor potential A1 (TRPA1) and GADPH were purchased from Abcam (Shanghai, China), incubated at 4°C overnight and washed in Western Wash Buffer for 5 min three times. The secondary antibody marked by horseradish peroxidase was incubated for 1 h at 4°C and washed in Western Wash Buffer for 5 min three times. Finally, the proteins were detected and observed using BeyoECL Plus Kit (Beyotime, Shanghai, China) and X-OMAT BT film (Carestream, USA).
Calcium imaging assay
RSC96 cells were treated with AITCR, seeded in 24-well plate, added Fura-2AM (Tocris bioscience, Bristol, UK) solution for 30 min at the room temperature, and washed in Ringer solution for 30 min in the dark. The Fura-2 loaded cells were exposed for 30s at the excitation wavelength distribution of 340nm and 380nm using a fluorescence microscope (Leica Microsystems, Weitzlar, Germany). The calculated F340/F380 ratio represented the relative calcium level.
Statistical analysis
All data were represented by the mean ± standard deviation (SD). SPSS13.0 analysis software (SPSS Inc., Chicago, USA) was used to perform t-test between groups. The difference was statistically significant at p<0.05.
Results
J147 reduced MWT of STZ-induced DPN rats in vivo
In order to study the effect of J147 on DPN, we detected MWT of rat models in vivo using Von Frey filaments at 10 μM and 100 μM of J147. As shown in Figure 1, we found that the thresholds of rats fed J147 were decreased as time went on. Besides, the threshold was significantly reduced in the 10 μMJ147 group compared to the control group, while it after feeding 100 μM of J147 also clearly lower than that 10 μM of J147.
J147 reduced MWT of STZ-induced DPN rats in vivo. The MWT was measured by Von Frey filaments at 1, 2, 3, 4 and 5 days after feeding J147.
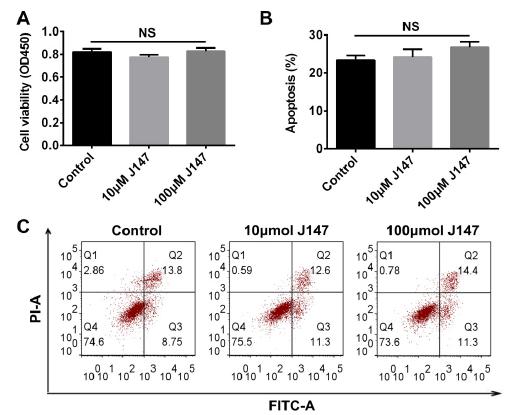
J147 had no obvious effect on cell viability and apoptosis
Subsequently, we examined the effect of J147 on RSC96 cells in vitro using MTT and flow cytometry. In Figure 2A, cell viability did not change in cells treated with J147. Similarly, there was no significant difference in apoptosis between the 3 groups (Figure 2 B and C).
J147 had no obvious effect on cell viability and apoptosis. Cell viability and apoptosis were measured by MTT and flow cytometry assay.
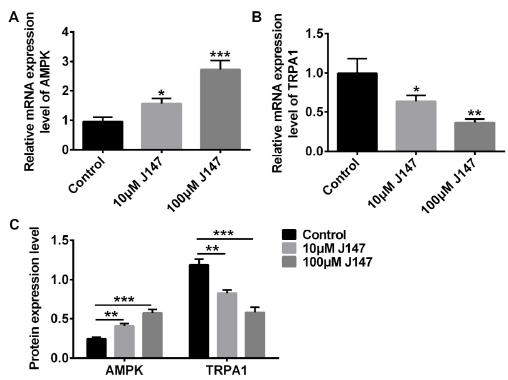
J147 increased AMPK expression and reduced TRPA1 expression
To study the molecular mechanism of the influence of J147 on DPN, we detected the expression levels of AMPK and TRPA1 using qRT-PCR and western blot assay. As show in Figure 3A, AMPK expression was clearly up-regulated in 10 μM and 100 μM of J147 treated cells, and the increase in the 100 μMJ147 group was more obvious. On the contrary, the expression of TRPA1 was down-regulated in the 10 μM and 100 μM group compared with the control group (Figure 3B). Subsequently, Figure 3C also suggested that J147 increased the protein expression level of AMPK, and decreased TRPA1 expression level.
J147 increased AMPK expression and reduced TRPA1 expression. The mRNA and protein expression levels of AMPK and TRPA1 were assessed by qRT-PCR and western blot.
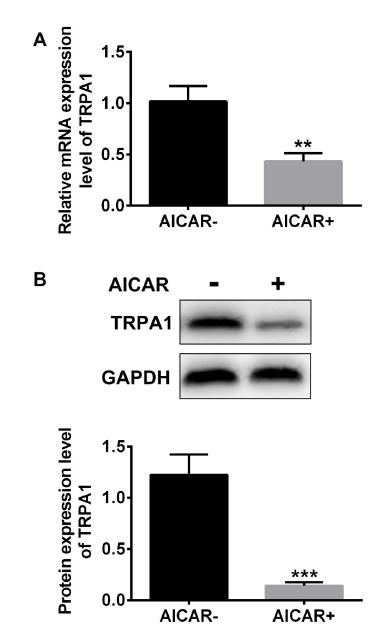
AMPK negative regulated TRPA1 expression in RSC96 cells
In this study, we used agonist AICAR to treated RSC96 cells, and detected TRPA1 expression. In Figure 4A, qRT-PCR results showed that TRPA1 expression level in the agonist AICAR group was significantly lower than that in the non-agonist group. Analogously, western blot analysis results also suggested that agonist AICAR remarkably decreased the protein expression level of TRPA1 compared with the AICAR- group (Figure 4B).
AMPK negative regulated TRPA1 in RSC96 cells. Cells were treated with AICAR. The mRNA and protein expression level of TRPA1 was assessed by qRT-PCR and western blot.
J147 reduced calcium reaction level in AITCR treated RSC96 cells
Finally, in order to investigate the function of TRPA1, RSC96 cells were treated with agonist AICAR and detected using calcium imaging assay. We found that calcium reaction level was significantly decreased in the 10 μM and 100 μM of J147 group than the control group, and the lowest level of calcium declined in the 100 μM of J147 group (Figure 5).
J147 reduced calcium reaction level in AITCR treated RSC96 cells. Cells were treated with AICAR. The calcium reaction level was detected by calcium imaging assay.
Discussion
Due to curcumin can relieve diabetic peripheral neuropathic pain1414 Banafshe HR, Hamidi GA, Noureddini M, Mirhashemi SM, Mokhtari R, Shoferpour M. Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol. 2014;723:202-6. doi: 10.1016/j.ejphar.2013.11.033.
https://doi.org/10.1016/j.ejphar.2013.11...
, and J147 as a derivative of curcumin, we concluded that it has a certain effect on DPN. Hence, in this study, we aimed to investigate the specific molecular mechanisms and effects of J147 on DPN through the establishment of DPN rat models, cell viability, apoptosis and calcium imaging assay. We found that J147reduced MWT in vivo, increased the mRNA and protein level of AMPK, reduced TRPA1 expression and calcium reaction level in AITCR treated RSC96 cells, and had no obvious effect on cell viability and apoptosis. Besides, AMPK negative regulated TRPA1 expression in RSC96 cells.
It is reported that J147, as a positive neurotrophic factor, could promote animal memory, and maintain the normal presence of synaptic proteins and the basic level of cognitive ability in transgenic mice1616 Chen Q, Prior M, Dargusch R, Roberts A, Riek R, Eichmann C, Chiruta C, Akaishi T, Abe K, Maher P, Schubert D. A novel neurotrophic drug for cognitive enhancement and Alzheimer's disease. PLoS One. 2011;6(12):e27865. doi: 10.1371/journal.pone.0027865.
https://doi.org/10.1371/journal.pone.002...
. In addition, J147 inhibits β-amyloid (Aβ) oligomerization and alleviates Aβ-induced cytotoxicity in neuronal cells2020 Kim K, Park K-S, Kim MK, Choo H, Chong Y. Dicyanovinyl-substituted J147 analogue inhibits oligomerization and fibrillation of ß-amyloid peptides and protects neuronal cells from ß-amyloid-induced cytotoxicity. Org Biomol Chem. 2015 Oct 7;13(37):9564-9. doi: 10.1039/c5ob01463h.
https://doi.org/10.1039/c5ob01463h...
. The underlying mechanism of mechanical hyperalgesia is the phenotypic transformation of Aβ fibers, thereby secreting neuropeptides expressed by small fibers2121 Zhang Y, Chen Y, Liedtke W, Wang F. Lack of evidence for ectopic sprouting of genetically labeled Aß touch afferents in inflammatory and neuropathic trigeminal pain. Mol Pain. 2015;11(1):1-9. doi: 10.1186/s12990-015-0017-2.
https://doi.org/10.1186/s12990-015-0017-...
.Because of DPN can produce corresponding sensory dysfunction and cause nerve damage, leading to loss of pain sensitivity in some areas2222 Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13(9):924-35. doi: 10.1016/s1474-4422(14)70102-4.
https://doi.org/10.1016/s1474-4422(14)70...
. Therefore, in this study, we constructed STZ-induced DPN rat models to simulate DPN patients, and detected their MWT to facilitate the understanding of the effects of J147 on DPN in vivo. The results showed that J147 significantly reduced MWT of rat, and 100μM of J147 had a better effect.
AMPK is an enzyme that controls the energy balance in cells, consisting of 3 subunits of α, βandγ. It can achieve energy balance via adjusting blood glucose levels, fatty acid oxidation and glycogen metabolism2323 Fryer LG, Carling D. AMP-activated protein kinase and the metabolic syndrome. Biochem Soc Trans. 2005;33(Pt 2):362-6. doi: 10.1042/bst0330362. PMID: 15787607.
https://doi.org/10.1042/bst0330362...
. The activity of AMPK is mainly regulated by intracellular AMP/ATP ratio. In the stress state with increased ratio, such as lack of nutrition, blood glucose deficiency, exercise, ischemia and hypoxia, AMPK activation can be induced2424 Cao S, Li B, Yi X, Chang B, Zhu B, Lian Z, Zhang Z, Zhao G, Liu H, Zhang H. Effects of exercise on AMPK signaling and downstream components to PI3K in rat with type 2 diabetes. Plos One. 2012;7(12):e51709. doi: 10.1371/journal.pone.0051709.
https://doi.org/10.1371/journal.pone.005...
. In our study, the results of qRT-PCR and western blot suggested that AMPK expression level was up-regulated in J147 treated cells, and could laterally reflect that J147 alleviated DPN in vitro.
TRPA1 is an instantaneous receptor-potential ion channel that was first discovered in the DRG and trigeminal ganglion neurons, and was cloned from the temperature sensitive neurons of the rats2525 Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ. Transient Receptor Potential Vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Mol Pain. 2010;6:15. doi: 10.1186/1744-8069-6-15.
https://doi.org/10.1186/1744-8069-6-15...
. It has been reported that blocking TRPA1 in the STZ-induced rodent acute diabetes model can reduce the high sensitivity of mechanical stimulation2626 Wei H, Hamalainen MM, Saarnilehto M, Koivisto A, Pertovaara A. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology. 2009;111(1):147-54. doi: 10.1097/ALN.0b013e3181a1642b.
https://doi.org/10.1097/ALN.0b013e3181a1...
. A large number of oxidative stress reactions produced during the metabolic process of diabetes can emerge pain, hyperalgesia and neurogenic inflammation through activating TRPA12727 Huang Q, Chen Y, Gong N, Wang YX. Methylglyoxal mediates streptozotocin-induced diabetic neuropathic pain via activation of the peripheral TRPA1 and Nav1.8 channels. Metabolism. 2016;65(4):463-74. doi: 10.1016/j.metabol.2015.12.002.
https://doi.org/10.1016/j.metabol.2015.1...
. Our results showed that J147 down-regulated the mRNA and protein expression level of TRPA1.
Subsequently, Wang et al.2828 Wang S, Kobayashi K, Kogure Y, Yamanaka H, Yamamoto S, Yagi H, Noguchi K, Dai Y. Negative regulation of TRPA1 by AMPK in primary sensory neurons as a potential mechanism of painful diabetic neuropathy. Diabetes. 2018;67(1):98-109. doi: 10.2337/db17-0503.
https://doi.org/10.2337/db17-0503...
found that there is a functional link between AMPK and TRPA1 in dorsal root ganglion (DRG) neurons. AMPK activation leads to the down-regulation of TRPA1 and its channel activity within a few minutes. High-glucose treatment reduces AMPK activity levels and increases agonist-induced TRPA1 currents in cultured DRG neurons2828 Wang S, Kobayashi K, Kogure Y, Yamanaka H, Yamamoto S, Yagi H, Noguchi K, Dai Y. Negative regulation of TRPA1 by AMPK in primary sensory neurons as a potential mechanism of painful diabetic neuropathy. Diabetes. 2018;67(1):98-109. doi: 10.2337/db17-0503.
https://doi.org/10.2337/db17-0503...
. Therefore, we studied the relationship between AMPK and TRPA1 by adding AICAR, and found that AMPK negatively regulated TRPA1 expression level in DPN cells model. In addition, activation of TRPA1 can cause calcium ions to permeate into cells2929 Wang YY, Chang RB, Hang NW, Mckemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008;283(47):32691-703. PMID: 18775987.. Then, we also detected calcium reaction level of AICAR-treated cells using calcium imaging assay. The results suggested that J147 reduced calcium reaction level in DPN cells, and were similar to those of previous studies.
Conclusions
Curcumin derivative J147 could ameliorate DPN through negative regulation AMPK on TRPA1in vivo and in vitro. These studies provide a more sufficient experimental basis for clinical treatment of DPN.
References
-
1El Boghdady NA, Badr GA. Evaluation of oxidative stress markers and vascular risk factors in patients with diabetic peripheral neuropathy. Cell Biochem Funct. 2012;30(4):328-34. doi:10.1002/cbf.2808.
» https://doi.org/10.1002/cbf.2808 -
2Wooten K. Clinical features and electrodiagnosis of diabetic peripheral neuropathy in the dysvascular patient. Phys Med Rehabil Clin N Am. 2009;20(4):657-76. doi: 10.1016/j.pmr.2009.06.011.
» https://doi.org/10.1016/j.pmr.2009.06.011 -
3Piao Y, Liang X. Chinese medicine in diabetic peripheral neuropathy: experimental research on nerve repair and regeneration. Evid Based Complement Alternat Med. 2012;2012:191632. doi: 10.1155/2012/191632.
» https://doi.org/10.1155/2012/191632 -
4Tesfaye S. Advances in the management of diabetic peripheral neuropathy. Curr Opin Support Palliat Care. 2009 Jun;3(2):136-43. doi: 10.1097/SPC.0b013e32832b7df5.
» https://doi.org/10.1097/SPC.0b013e32832b7df5 -
5Martin CL, Albers J, Herman WH, Cleary P, Waberski B, Greene DA, Stevens MJ, Feldman EL. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care. 2006;29(2):340. PMID: 16443884.
-
6Gutierrez-Alvarez AM, Beltrán-Rodríguez J, Moreno CB. Antiepileptic drugs in treatment of pain caused by diabetic neuropathy. J Pain Symptom Manage. 2007 Aug;34(2):201-8 doi: 10.1016/j.jpainsymman.2006.10.023.
» https://doi.org/10.1016/j.jpainsymman.2006.10.023 -
7De CV, Rajan V, Steenbergen W, He DAV, Buschman HP. Effect and safety of spinal cord stimulation for treatment of chronic pain caused by diabetic neuropathy. J Diabetes Complications. 2009 Jan-Feb;23(1):40-5. doi: 10.1016/j.jdiacomp.2007.08.002.
» https://doi.org/10.1016/j.jdiacomp.2007.08.002 -
8Dellon AL. The Dellon approach to neurolysis in the neuropathy patient with chronic nerve compression. Handchir Mikrochir Plast Chir. 2008 Dec;40(6):351-60. doi: 10.1055/s-2008-1039216. PMID:19051159.
» https://doi.org/10.1055/s-2008-1039216 -
9Lu You, Lu Ying-li. Progress in pathogenesis of diabetic peripheral neuropathy. J Shanghai Jiaotong Univ. 2017;37(10):1441. doi: 10.3969/j.issn.1674-8115.2017.10.027.
» https://doi.org/10.3969/j.issn.1674-8115.2017.10.027 -
10Guastella V, Mick G. Strategies for the diagnosis and treatment of neuropathic pain secondary to diabetic peripheral sensory polyneuropathy. Diabetes Metab. 2009;35(1):12-9. doi: 10.1016/j.diabet.2008.09.003.
» https://doi.org/10.1016/j.diabet.2008.09.003 -
11Lakeybeitia J, González Y, Doens D, Stephens DE, Santamaría R, Murillo E, Gutiérrez M, Fernández PL, Rao KS, Larionov OV. Assessment of novel curcumin derivatives as potent inhibitors of inflammation and amyloid-ß aggregation in Alzheimer's disease. J Alzheimers Dis. 2017;60(s1):S59-S68. doi: 10.3233/JAD-170071.
» https://doi.org/10.3233/JAD-170071 -
12Baloch Z, Yasmeen N, Li Y, Zhang W, Lu H, Wu X, Xia X, Yang S. Knowledge and awareness of cervical cancer, human papillomavirus (HPV), and HPV vaccine among HPV-infected chinese women. Med Sci Monit. 2017;23:4269-77. PMID: 28867818.
-
13Srinivasan M. Effect of curcumin on blood sugar as seen in a diabetic subject. Indian J Med Sci. 1972;26(4):269-70. PMID: 4637293.
-
14Banafshe HR, Hamidi GA, Noureddini M, Mirhashemi SM, Mokhtari R, Shoferpour M. Effect of curcumin on diabetic peripheral neuropathic pain: possible involvement of opioid system. Eur J Pharmacol. 2014;723:202-6. doi: 10.1016/j.ejphar.2013.11.033.
» https://doi.org/10.1016/j.ejphar.2013.11.033 -
15Cocorocchio M, Baldwin AJ, Stewart B, Kim L, Harwood AJ, Thompson CRL, Andrews PLR, Williams RSB. Curcumin and derivatives function through protein phosphatase 2A and presenilin orthologues in Dictyostelium discoideum. Dis Model Mech. 2018 Jan 29;11(1). doi: 10.1242/dmm.032375.
» https://doi.org/10.1242/dmm.032375 -
16Chen Q, Prior M, Dargusch R, Roberts A, Riek R, Eichmann C, Chiruta C, Akaishi T, Abe K, Maher P, Schubert D. A novel neurotrophic drug for cognitive enhancement and Alzheimer's disease. PLoS One. 2011;6(12):e27865. doi: 10.1371/journal.pone.0027865.
» https://doi.org/10.1371/journal.pone.0027865 -
17Goldberg J, Currais A, Prior M, Fischer W, Chiruta C, Ratliff E, Daugherty D, Dargusch R, Finley K, Esparza-Molto PB, Cuezva JM, Maher P, Petrascheck M, Schubert D. The mitochondrial ATP synthase is a shared drug target for aging and dementia. Aging Cell. 2018;17(2). doi: 10.1111/acel.12715.
» https://doi.org/10.1111/acel.12715 -
18Lian L, Xu Y, Zhang J, Yu Y, Zhu N, Guan X, Huang H, Chen R, Chen J, Shi G, Pan J. Antidepressant-like effects of a novel curcumin derivative J147: Involvement of 5-HT1A receptor. Neuropharmacology. 2018;135:506-13. doi: 10.1016/j.neuropharm.2018.04.003.
» https://doi.org/10.1016/j.neuropharm.2018.04.003 -
19Prior M, Dargusch R, Ehren JL, Chiruta C, Schubert D. The neurotrophic compound J147 reverses cognitive impairment in aged Alzheimer's disease mice. Alzheimers Res Ther. 2013;5(3):25. doi: 10.1186/alzrt179.
» https://doi.org/10.1186/alzrt179 -
20Kim K, Park K-S, Kim MK, Choo H, Chong Y. Dicyanovinyl-substituted J147 analogue inhibits oligomerization and fibrillation of ß-amyloid peptides and protects neuronal cells from ß-amyloid-induced cytotoxicity. Org Biomol Chem. 2015 Oct 7;13(37):9564-9. doi: 10.1039/c5ob01463h.
» https://doi.org/10.1039/c5ob01463h -
21Zhang Y, Chen Y, Liedtke W, Wang F. Lack of evidence for ectopic sprouting of genetically labeled Aß touch afferents in inflammatory and neuropathic trigeminal pain. Mol Pain. 2015;11(1):1-9. doi: 10.1186/s12990-015-0017-2.
» https://doi.org/10.1186/s12990-015-0017-2 -
22Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13(9):924-35. doi: 10.1016/s1474-4422(14)70102-4.
» https://doi.org/10.1016/s1474-4422(14)70102-4 -
23Fryer LG, Carling D. AMP-activated protein kinase and the metabolic syndrome. Biochem Soc Trans. 2005;33(Pt 2):362-6. doi: 10.1042/bst0330362. PMID: 15787607.
» https://doi.org/10.1042/bst0330362 -
24Cao S, Li B, Yi X, Chang B, Zhu B, Lian Z, Zhang Z, Zhao G, Liu H, Zhang H. Effects of exercise on AMPK signaling and downstream components to PI3K in rat with type 2 diabetes. Plos One. 2012;7(12):e51709. doi: 10.1371/journal.pone.0051709.
» https://doi.org/10.1371/journal.pone.0051709 -
25Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ. Transient Receptor Potential Vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Mol Pain. 2010;6:15. doi: 10.1186/1744-8069-6-15.
» https://doi.org/10.1186/1744-8069-6-15 -
26Wei H, Hamalainen MM, Saarnilehto M, Koivisto A, Pertovaara A. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology. 2009;111(1):147-54. doi: 10.1097/ALN.0b013e3181a1642b.
» https://doi.org/10.1097/ALN.0b013e3181a1642b -
27Huang Q, Chen Y, Gong N, Wang YX. Methylglyoxal mediates streptozotocin-induced diabetic neuropathic pain via activation of the peripheral TRPA1 and Nav1.8 channels. Metabolism. 2016;65(4):463-74. doi: 10.1016/j.metabol.2015.12.002.
» https://doi.org/10.1016/j.metabol.2015.12.002 -
28Wang S, Kobayashi K, Kogure Y, Yamanaka H, Yamamoto S, Yagi H, Noguchi K, Dai Y. Negative regulation of TRPA1 by AMPK in primary sensory neurons as a potential mechanism of painful diabetic neuropathy. Diabetes. 2018;67(1):98-109. doi: 10.2337/db17-0503.
» https://doi.org/10.2337/db17-0503 -
29Wang YY, Chang RB, Hang NW, Mckemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008;283(47):32691-703. PMID: 18775987.
-
Financial source:
none
-
1
Research performed at Department of Prescription, Basic Medical College, Shanxi University, Traditional Chinese Medicine, China.
Publication Dates
-
Publication in this collection
June 2018
History
-
Received
11 Feb 2018 -
Reviewed
10 Apr 2018 -
Accepted
12 May 2018