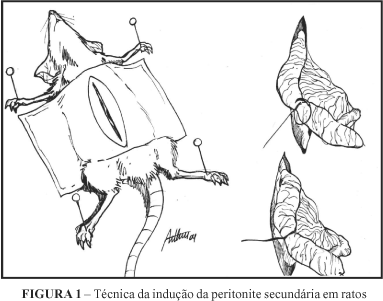
OBJECTIVES: The acute peritonitis is an important cause of sepsis and death on intensive care units and surgery. The treatment must include: systemical use of antibiotics, drainage of abscess and restauration of gastrointestinal integrity. The topical use of antibiotics in the peritoneal cavity is controversial. The aim of this study was to evaluate the use of topical use of ampicilin/sulbactam in the treatment of peritonitis. METHODS: We measured the plasmatic levels of nitric oxide, count of eosinophils, lymphocytes, monocytes, and neutrophils in blood and peritoneal cavity, using a model of peritonitis in rats (transfixation and ligature of cecum). Twenty four Wistar rats were divided in 4 groups (n=6 each). group A: induction of peritonitis with ligature of cecum and topical treatment with saline; group B: induction of peritonitis with ligature of cecum and topical treatment with ampicilin/sulbactam; group C: transfixation of cecum; group D: laparotomy and peritoneal exsudate + blood sample. The transfixation-ligture of cecum remained for 24 hs before treatment. A relaparotomy was performed in 18 rats and peritoneal exsudate/blood were collected. Dosage of Nitric oxide, count of eosinophil, lynphocytes, monocytes, and neutrophils in blood and peritoneal exsudte were done. RESULTS: The difference was not significant in the levels of nitric oxide, eosinophil, lynphocytes, monocytes, and neutrophils in blood and peritoneal exsudate (p > 0,05) among the studied groups. CONCLUSON: The use of ampicilin associated to sulbactam via intraperitoneal in rats with fecal peritonitis did not change survival.; the levels of plama nitric oxide, count of eosinophil, lynphocytes, monocytes, and neutrophils in blood and peritoneal exsudate were not affected.
Peritonite; Antibióticos; Ratos