Abstracts
With the aim of determining the possible toxicity of amitraz after its prolonged use in horses, six English Thoroughbred horses received intravenous injections of amitraz (0.05, 0.10 or 0.15 mg/kg) weekly for four months, constituting the experimental group. Eight other animals (control group), via the same route following the same drug administration schedule and period of time, received the vehicle, dimethylformamide. At the end of this period, blood was collected from all the animals, and a comparison was made of the means of the values obtained for the various blood analyses: complete hemogram, alkaline phosphatase, gamma-glutamyltransferase, blood urea nitrogen, lactate dehydrogenase, aspartate aminotransferase, creatine phosphokinase, glucose, albumin, total protein, creatinine, Na+ , K+, Cl- and CO2. The results for the biochemical characteristics showed that only the mean value for urea of the animals submitted to treatment with amitraz was significantly different than the mean value obtained for the control group. The analyses of the hematological characteristics showed that no significant differences between groups were observed. Similarly, the measurement of blood electrolyte levels demonstrated that long-term treatment with amitraz did not cause significant changes in the variables analyzed. The results indicate that amitraz, given in the doses employed in this study, did not show signs of inducing toxic effects in vital organs, even after prolonged administration.
Horse; English Thoroughbred; amitraz; toxicity; blood characteristics; electrolytes
Estudou-se a possível toxicidade do uso prolongado do amitraz em eqüinos utilizando-se seis éguas Puro Sangue Inglês, as quais receberam injeções intravenosas de amitraz (0,05, 0,10 ou 0,15 mg/kg) semanalmente, durante quatro meses (grupo experimental). Outros oito animais (grupo-controle), seguindo o mesmo esquema de administração e período, receberam o veículo dimetilformamida. Ao final desse período colheram-se amostras de sangue dos animais e compararam-se as médias dos valores obtidos para as seguintes variáveis do sangue: hemograma completo, fosfatase alcalina, gama-glutamiltransferase, nitrogênio uréico, desidrogenase láctica, aspartato aminotransferase, creatina fosfoquinase, glucose, albumina, proteína total, creatinina, Na+ , K+, Cl- e CO2. Apenas as médias dos valores de nitrogênio uréico dos animais submetidos ao tratamento com amitraz foram diferentes das médias dos valores obtidos para o grupo-controle. As análises das características hematimétricas mostraram que não houve diferenças significativas quando se compararam os dois grupos. As dosagens de eletrólitos no sangue também demonstraram que o tratamento prolongado com amitraz não causou alterações significativas nas características analisadas. O amitraz na dosagem empregada não induziu efeitos tóxicos sobre órgãos vitais, mesmo após administração prolongada.
Cavalo Puro Sangue Inglês; amitraz; toxicidade; variáveis sangüíneas; eletrólitos
Toxicological evaluation of long-term intravenous administration of amitraz in horses
[Avaliação toxicológica da administração intravenosa crônica de amitraz em eqüinos]
A. Queiroz-Neto1, F.H.F. D'Angelis1, J.D. Harkins2, T. Tobin2
1Faculdade de Ciências Agrárias e Veterinárias UNESP Campus de Jaboticabal
Via de Acesso Professor Paulo Donato Castellane km 5
14884-900 - Jaboticabal, SP
2Gluck Equine Research Center, University of Kentucky, Lexington, KY, USA
Recebido para publicação em 8 de abril de 2001
Recebido para publicação, após modificações, em 8 de abril de 2002
E-mail: aqueiroz@fcav.unesp.br
ABSTRACT
With the aim of determining the possible toxicity of amitraz after its prolonged use in horses, six English Thoroughbred horses received intravenous injections of amitraz (0.05, 0.10 or 0.15 mg/kg) weekly for four months, constituting the experimental group. Eight other animals (control group), via the same route following the same drug administration schedule and period of time, received the vehicle, dimethylformamide. At the end of this period, blood was collected from all the animals, and a comparison was made of the means of the values obtained for the various blood analyses: complete hemogram, alkaline phosphatase, g-glutamyltransferase, blood urea nitrogen, lactate dehydrogenase, aspartate aminotransferase, creatine phosphokinase, glucose, albumin, total protein, creatinine, Na+ , K+, Cl- and CO2. The results for the biochemical characteristics showed that only the mean value for urea of the animals submitted to treatment with amitraz was significantly different than the mean value obtained for the control group. The analyses of the hematological characteristics showed that no significant differences between groups were observed. Similarly, the measurement of blood electrolyte levels demonstrated that long-term treatment with amitraz did not cause significant changes in the variables analyzed. The results indicate that amitraz, given in the doses employed in this study, did not show signs of inducing toxic effects in vital organs, even after prolonged administration.
Keywords: Horse, English Thoroughbred, amitraz, toxicity, blood characteristics, electrolytes
RESUMO
Estudou-se a possível toxicidade do uso prolongado do amitraz em eqüinos utilizando-se seis éguas Puro Sangue Inglês, as quais receberam injeções intravenosas de amitraz (0,05, 0,10 ou 0,15 mg/kg) semanalmente, durante quatro meses (grupo experimental). Outros oito animais (grupo-controle), seguindo o mesmo esquema de administração e período, receberam o veículo dimetilformamida. Ao final desse período colheram-se amostras de sangue dos animais e compararam-se as médias dos valores obtidos para as seguintes variáveis do sangue: hemograma completo, fosfatase alcalina, g-glutamiltransferase, nitrogênio uréico, desidrogenase láctica, aspartato aminotransferase, creatina fosfoquinase, glucose, albumina, proteína total, creatinina, Na+ , K+, Cl- e CO2. Apenas as médias dos valores de nitrogênio uréico dos animais submetidos ao tratamento com amitraz foram diferentes das médias dos valores obtidos para o grupo-controle. As análises das características hematimétricas mostraram que não houve diferenças significativas quando se compararam os dois grupos. As dosagens de eletrólitos no sangue também demonstraram que o tratamento prolongado com amitraz não causou alterações significativas nas características analisadas. O amitraz na dosagem empregada não induziu efeitos tóxicos sobre órgãos vitais, mesmo após administração prolongada.
Palavras-chave: Cavalo Puro Sangue Inglês, amitraz, toxicidade, variáveis sangüíneas, eletrólitos
INTRODUCTION
Amitraz is a formamidine acaricide widely utilized in veterinary medicine. In horses, the use of amitraz is customarily not indicated (Smith, 1994), because of the incidence of serious cases of colic after spray treatment. In spite of this, in some tropical countries such as Brazil, amitraz continues to be quite utilized in horses, since it is an acaricide that is very effective and inexpensive. However, in Brazil there are several reports pointing out undue accidents with its use in horses, such as the occurrence of 18 cases of horses with impaction of the large intestine after two to three days of bath applications containing amitraz (Botteon et al., 1998) among 64 cases of acute abdomen attended at the Veterinary Hospital of the Universidade Federal Rural do Rio de Janeiro in 1994 and 1995. It is worth noting also that the Veterinary Hospital of FCAV/UNESP in Jaboticabal has been frequently attending horses intoxicated by this drug.
Besides the acaricidal and insecticidal effects, amitraz has a quite complex pharmacological activity in mammals. In various species of animals, the common signs of acute intoxication are depression of the central nervous system (CNS) and episodes of hyperexcitability, hypotension, bradycardia, hypothermia, hyperglycemia and in some species, alterations in water balance, evidenced by hemoconcentration (Bonsall & Turnbull, 1983).
The signs described above could be related to the capacity of formamidines to inhibit monoamine oxidase (Aziz & Knowles, 1973; Benezet & Knowles, 1976), block the synthesis of prostaglandin E2 (Yim et al., 1978), have a local anesthetic effect (Chinn et al., 1977) and, what appears to be the principal mechanism of action, stimulate a2-adrenergic receptors (Costa & Murphy, 1987).
In investigating the antinociceptive effect of amitraz in rats and mice, by comparing it with the effect obtained with xylazine and detomidine, two a2-adrenergic agonists utilized largely in veterinary medicine, Queiroz-Neto et al. (1994) concluded that the effect of amitraz is comparable in potency to that of the traditional a2-adrenergic agonists, being also blocked by idazoxam, an a2-adrenergic blocker. Similarly, Harkins et al. (1997) by studying the sedative effect of the same drugs in a behavior stall, observed that amitraz as well as xylazine and detomidine caused a diminution of the spontaneous locomotor activity of horses, characterizing their sedative effect. Furthermore in this case, the effect of amitraz was also reversed by the administration of an a2-adrenergic blocker (yohimbine).
Queiroz-Neto et al. (1998) in considering the potential therapeutic use of amitraz as an a2-adrenergic agonist investigated the sedative and antinociceptive effect of this drug after an IV administration at doses of 0.05, 0.10 and 0.15 mg/kg in English Thoroughbred (ETB) horses. The results obtained indicated that amitraz shows a more potent and long-lasting sedative effect in horses when compared to xylazine. The antinociceptive effect, determined by the increase in the skin twitch reflex latency, was observed only at the highest dose of amitraz, indicating that the principal action of this drug is the sedative effect.
More recently, the same group of investigators (Queiroz-Neto et al., 2000) compared the effects of amitraz (0.1mg/kg IV) and xylazine (1 mg/kg IV) on physiological variables in horses. Amitraz caused a significant reduction in cardiac activity, respiratory frequency and bowel movements, but these effects were not as intense as those by xylazine. Amitraz still caused a significant relaxation of the smooth musculature of the rectum and an apparent increase in sweating and frequency of chewing hay. Rectal temperature was not influenced by amitraz. It was concluded that amitraz, after acute drug administration and in the dose utilized, did not cause relevant side effects in horses.
The effect of amitraz on plasma concentrations of glucose and insulin was studied in dogs by Hsu & Schaffer (1988). These authors observed that, at least in part, the hyperglycemic effect could have been due to its capacity to inhibit insulin release. Subsequently, investigators from the same laboratory (Smith et al., 1990) reported that the inhibition of insulin release in rats is mediated through the stimulation of a2 adrenergic receptors.
More recently, Di Filippo et al. (2000) proposed that amitraz may have an antiinflammatory effect. These authors examined the possible antiinflammatory action of this drug in cultured C3H mouse spleen cells stimulated with concanavalin A or lipopolysaccharide.
However, there are still several questions to be answered on the origin of the pharmacological effects of amitraz. Nonetheless, it is commonly believed by the majority of those investigating amitraz that these effects are partially derived from the stimulation of adrenergic receptors.
The aim of the present study was to determine the possible toxicity of amitraz after its prolonged use in ETB horses, by examining its effect on hematological parameters and on serum levels of enzyme activities and other biochemical parameters, whose alteration could indicate deleterious effects in organs or organic systems.
MATERIALS AND METHODS
Changes in the values of physiological variables caused by the IV administration of amitraz (Sintesul, Pelotas, RS, Brazil) were examined utilizing 14 ETB mares belonging to the experimental herd of the Department of Veterinary Sciences at the University of Kentucky, Lexington, KY, USA.
The evaluation of the toxicity of amitraz was conducted by comparing the mean values obtained for the blood variables of animals that received only vehicle IV (dimethylformamide, N=8), with those of animals that received amitraz IV (0.05, 0.10 or 0.15 mg/kg) per week (a different dose every week, consecutively) for four months (N=6). Blood samples were collected at the end of the four months period, one week after the last amitraz administration.
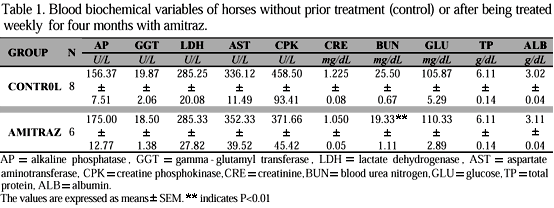
The parameters examined were: a complete hemogram, alkaline phosphatase (AP), g-glutamyltransferase (GGT), urea nitrogen (BUN), lactate dehydrogenase (LDH), aspartate aminotransferase (AST), creatine phosphokinase (CPK), glucose (GLU), albumin (ALB), total protein (TP), creatinine (CRE), Na+ , K+, Cl- and CO2. These laboratory tests were carried out by the Veterinary Hospital of Haygard Davidson McGee Associates, PSC, Lexington, KY, USA.
The hematology tests were performed utilizing a Serona Baker System cell counter, with the differential count done manually. The biochemical tests were performed with the help of an Roche Cobas Mira Chemical Analyzer, except for the electrolytes which were measured using a Beckman E4A Electrolyte Analyzer. The results were analyzed for statistical significance by Student's non-paired t-test.
RESULTS AND DISCUSSION
The hematological and serum biochemical assays are frequently utilized to determine the toxicity of substances introduced into living organisms. According to Silveira (1988), biochemical tests are utilized to estimate the normal functioning of various organs by cells involved mainly in the processes of synthesis, secretion, excretion and storage of certain substances. Therefore, plasma levels of various substances and enzymes can furnish assistance for the better and earlier evaluation of drug effect on the functional state of an organ.
Elevated transaminase (ALT and AST), AP and GGT activities suggest the presence of hepatocellular damage (Cornelius, 1989; Kramer, 1989). Similarly, Kramer (1989) suggested that increasis in serum enzyme activities for CPK, LDH and AST, would be indicators of myopathies of skeletal and cardiac muscle.
Besides enzymes, other substances which variation in concentration in plasma or serum could suggest pathological changes in organs include: glucose availability in blood which is intimately connected with the functioning of the liver (Coles, 1984); proteins, particularly albumin decreased levels, as according to Cornelius (1989), which could be associated with chronic hepatic changes; and urea and creatinine blood levels which furnish a good source of information about renal function (Silveira, 1988).
Acute administration of amitraz in animals can cause alterations in various blood parameters. In horses, these changes include an increase in RBCs, hematocrit, hemoglobin, total protein, urea, indirect bilirubin and glucose, and a diminution of potassium levels (Roberts & Seawright, 1983; Auer et al., 1984). Such parameters, according to these authors, with the exception of those related to the RBC indices and to protein concentration, were associated with the occurrence of dehydration and consequently hemoconcentration, and have not been previously described. The results presented in Table 1 relating to blood biochemical parameters, showed that only the mean value of urea in animals treated with amitraz was significantly different from the mean value obtained for the control group. Thus, the results obtained indicate that the chronic administration of amitraz in the doses and schedule studied did not inflict organic changes in the animals, as could be detected by the biochemical assays utilized for hepatic, renal and muscular functions. In relation to the observed difference for urea levels, it should be noted that although the value obtained for the group that received amitraz was significantly lower than that of the control group, both were within the limits of variation considered normal for this animal species.
With regard to the results in plasma glucose concentrations, our study, although showing a slight increase in animals treated with amitraz, could not evidentiate a statistically significant difference, therefore disagreeing with the results reported by Hsu & Schaffer (1988), who detected a hyperglycemic effect of amitraz in dogs atributing it to the capacity of this pesticide to inhibit insulin release.
The analyses of the hematological parameters displayed in Table 2 demonstrate that no statistically significant differences occurred between the mean values obtained for the several variables in the experimental and control groups. These results suggest the absence of adverse effects that could be detected by the study of a hemogram in animals that received amitraz weekly for four months.
Similarly, electrolyte levels in blood, shown in Table 3, also demonstrate that the prolonged treatment with amitraz did not cause significant changes in the parameters analyzed.
Although the results presented in this work indicate that the administration of amitraz did not cause pathological changes, eventual deleterious effects could occur in the animal, taking into account that the mechanism of action of this substance is quite complex, deserving further studies which will contribute to its elucidation. The studies carried out regarding amitraz will contribute to a more rational use of this drug, in a manner which will attenuate or even avoid the occurrence of unwanted effects in horses.
Once sedative and/or analgesic drugs are no longer customarily used for daily medication, eventually, the use of amitraz may be considered as a safe sedative and as a drug proven to be free of relevant side effects in horses, at the doses and with the schedule of administration described in the present study.
CONCLUSION
In light of the results obtained, it can be concluded that with the administration of amitraz in doses and in the treatment regimen employed in this study, neither apparent serious side effects, nor any indications of toxic effects on vital organs, even after a prolonged period of administration, were observed.
ACKNOWLEDGEMENTS
This work was supported by Brazilian research foundations Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
- AUER, D.E.; SEAWRIGHT, A.A.A.; POLLIT, C.C. et al. Illness in horses following spraying with amitraz. Aust. Vet. J., v.61, p.257-259, 1984.
- AZIZ, S.A.; KNOWLWS, C.O. Inhibition of monoamine oxidase by the pesticide chlordimeform and related compounds. Nature, v.242, p.417-418, 1973.
- BENEZET, H.J.; KNOWLES, C.O. Inhibition of rat brain monoamine oxidase by formamidines and related compounds. Int. J. Neuropharmacol, v.15, p.369-373, 1976.
- BONSALL, S.L.; TURNBULL, G.J. Extrapolation from safety data to management of poisoning with reference to amitraz (a formamidine pesticide) and xilene. Hum. Toxicol., v.2, p.587, 1983.
- BOTTEON, P.T.L.; BOTTEON, R.C.C.M.; SARTORI, F. Cólica em eqüinos associada ao uso de amitraz. Rev. Bras. Med. Vet., v.20, p.219-220, 1998.
- CHINN, C.; LUND, A.E.; YIM, G.K.W. Central action of lidocaine and the pesticide chlordimeform. Neuropharmacology, v.16, p.867-871, 1977.
- COLES, E.H. Patologia clínica veterinária 3.ed. São Paulo: Manole, 1984.
- CORNELIUS, C.E. Liver function. In: KANEKO, J.J. Clinical biochemistry of domestic animals 4.ed. San Diego: Academic Press, 1989. p.364-397.
- COSTA, L.G.; MURPHY, S.D. Interaction of the pesticide chlordimeform with adrenergic receptors in mouse brain: an in vitro study. Arch. Toxicol., v.59, p.323-327, 1987.
- Di FILIPPO, PA.; SOUZA, R.V.; SANTANA, G.C. et al. Atividade antinflamatória in vitro do amitraz e do solvente arol em células esplênicas de camundongos C3H. In: CONGRESSO LATINO AMERICANO DE FARMACOLOGIA, 16, 2000, Águas de Lindóia. Anais..São Paulo, p.312. (Resumo).
- HARKINS, J.D.; QUEIROZ-NETO, A.; MUNDY, G.D. et al. Development and characterization of an equine behavior chamber and the effect of amitraz and detomidine on spontaneous locomotor activity. J. Vet. Pharmacol. Ther, v.20, p.396-401, 1997.
- HSU, W.H.; SCHAFFER, D.D. Effects of topical application of amitraz on plasma glucose and insulin concentrations in dogs. Am. J. Vet. Res., v.49, p.130-131, 1988.
- KRAMER, J.W. Clinical enzimology. In: KANEKO, J.J. Clinical biochemistry of domestic animals 4.ed. San Diego: Academic Press, 1989, p. 338-363.
- QUEIROZ-NETO, A.; JUANG, S.J.; SOUZA, K.R. et al. Antinociceptive effect of amitraz in mice and rats. Braz. J. Med. Biol. Res., v.27, p.1407-1411, 1994.
- QUEIROZ-NETO, A.; ZAMUR, G.; GONÇALVES, S.C. et al. Characterization of the antinociceptive and sedative effects of amitraz in horses. J .Vet. Pharmacol. Therap, v.21, p.400-405, 1998.
- QUEIROZ-NETO, A.; CARREGARO, A.B.; ZAMUR, G. et al. Effect of amitraz and xylazine on some physiological variables of horses. Arq. Bras. Med. Vet. Zootec., v.52, p.1-6, 2000.
- ROBERTS, M.C.; SEAWRIGHT, A.A. Experimental studies of drugs-induced impaction colic in the horse. Equine Vet. J., v.15, p.222-228, 1983.
- SILVEIRA, J. M. Patologia clínica veterinária: teoria e interpretação Rio de Janeiro, 1988. 196p.
- SMITH, B.E.; HSU, W.H.; YANG, P.C. Amitraz-induced glucose intolerance in rats: antagonism by yohimbine but not by prazosin. Arch. Toxicol., v.64, p.680-683, 1990.
- SMITH, S.E. Treatment of mange in horses. Vet. Rec., v.134, p.508, 1994.
Publication Dates
-
Publication in this collection
16 Dec 2002 -
Date of issue
Aug 2002
History
-
Accepted
08 Apr 2002 -
Received
08 Apr 2001